Example of a good Doppler.
Uterine artery Doppler
The uterine artery (UtA) Doppler assesses the placental flow on the maternal side of the circulation and is able to identify impaired placentation through abnormal flow velocity and increased resistance. The artery is identified at the cervicocorporeal junction with the help of color flow either transabdominally or transvaginally. It commonly crosses the external iliac artery anteriorly and superiorly, and is seen to ascend towards the uterine body. Both the left and right UtA PI should be measured separately and the mean is reported. The presence of notching, unilateral or bilateral, should be noted[4] (see Chapter 21). There appears to be a progressive decrease in UtA mean PI from 11 weeks through to 34 weeks’ gestation, and stabalizes until term[5].
UtA Doppler is used as a screening tool for FGR and pre-eclampsia in high-risk pregnancies (see Chapter 21). Abnormal UtA Doppler in the first or second trimesters is associated with perinatal complications. A recent systematic review reported that an abnormal 1st trimester UtA Doppler (PI or RI >90th centile or notching) is a highly specific test for predicting early-onset pre-eclampsia and early-onset FGR[6]. In the second trimester (20–24 weeks’ gestation), UtA PI is not influenced by age, weight, height, method of conception, smoking, family history of pre-eclampsia, or pre-existing medical disorders such as chronic hypertension, diabetes mellitus, systemic lupus erythematosus or antiphospholipid syndrome. UtA Doppler is significantly higher in women of Afro-Caribbean racial origin and lower in South and East Asian women compared with Caucasians, higher in multiparous women with a previous history of pre-eclampsia and SGA, and decreases with gestational age. Thus, these characteristics need to be adjusted in a screening programme utilizing UtA Doppler. An abnormal UtA PI is also related to the severity of pre-eclampsia as there is a significant inverse association between uterine PI and the gestational age at delivery and fetal birthweight[7].
UA Doppler
The UA Doppler evaluates placental function and is the most commonly used parameter in the monitoring of fetal condition in high-risk pregnancies. Its use is associated with a reduction in perinatal death from 1.7% to 1.2%, with fewer inductions of labor and cesarean sections in high-risk pregnancies[8]. In a normal pregnancy, there should be a low-resistance system with forward flow throughout the cardiac cycle. There is a significant difference in the flow indices at the fetal end and placental end of the umbilical cord, with higher resistance in the fetal end. Thus, this test is best performed on a free loop of umbilical cord using color Doppler for consistency[4].
With increasing placental dysfunction, there is progressive change from a normal waveform, to increased vessel resistance flow, to absent end-diastolic flow (EDF), and/or reversed EDF (see Chapter 21). As discussed in Chapter 21, changes in the UA waveforms and resistance are used in the management of SGA/FGR. Similarly, an abnormal UA Doppler in other high-risk pregnancies, such as postdates, RFM or maternal diabetes, can be used to initiate further fetal surveillance testing, including other Doppler parameters and/or CTG. In cases of multiple pregnancies, there is a risk of assigning the wrong cord loop to the fetus, thus it is best to sample the UA just distal to the umbilical cord insertion at the fetal end[4]. It is important to remember that this segment of the cord has higher resistance compared to the placental end. Although there are published reference ranges for UA PI at different parts of the UA[9], it may be more useful to follow the umbilical cord and measure PI at different segments along the cord to obtain an average UA PI for interpretation of results when there is raised UA PI at the fetal end.
Middle cerebral artery
The fetal middle cerebral artery (MCA) is short and straight, and its Doppler flow indices are commonly used in the assessment of FGR (see Chapter 21 and in the diagnosis and management of fetal anemia (Chapter 15)). In addition to the techniques described above for obtaining reproducible Doppler signals, the gate for MCA Doppler assessment should be placed at the proximal third of the MCA, close to its origin from the internal carotid artery[4] (Figure 22.2). The angle between the ultrasound beam and direction of blood flow should be kept as close as possible to 0o and angle corrections should be avoided. This is especially important when using MCA in the diagnosis of fetal anemia as peak systolic velocity (PSV) changes according to the angle of insonation, and systolic velocity decreases with distance from the point of origin of MCA.
The exact mechanisms of reduced MCA resistance in FGR are not fully understood and are likely to be a combination of centralization of brain blood flow due to increased left ventricular cardiac output, and hypoxemia-induced vasodilatation in cerebral vasculature, resulting in the “brain sparing” effect. The proportional change may be first detected by the cerebroplacental ratio (see Chapter 21). An abnormal MCA Doppler increases the likelihood of neonatal compromise, and warrants further tests of fetal wellbeing, but does not necessarily mean an immediate need for delivery.
MCA Doppler is also used in the identification and management of fetal anemia secondary to conditions such as maternal–fetal alloimmunization, parvovirus infection, fetal–maternal hemorrhage, unexplained hydrops or twin anemia-polycythemia sequence. Fetuses with anemia have a high cardiac output and decreased blood viscosity, resulting in high blood-flow velocities in the MCA, giving an elevated MCA-PSV. The PSV can be measured with the autotrace function or using manual calipers of the maximum velocity (Figures 22.2 and 22.3). Fetal anemia that requires intervention is commonly diagnosed when the MCA-PSV is >1.5 multiples of the median for the gestation[10].
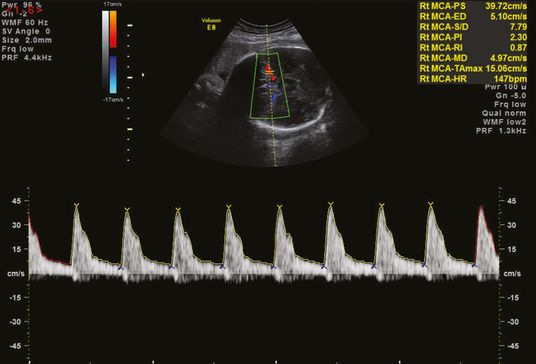
Middle cerebral artery peak systolic velocity using the autotrace function.
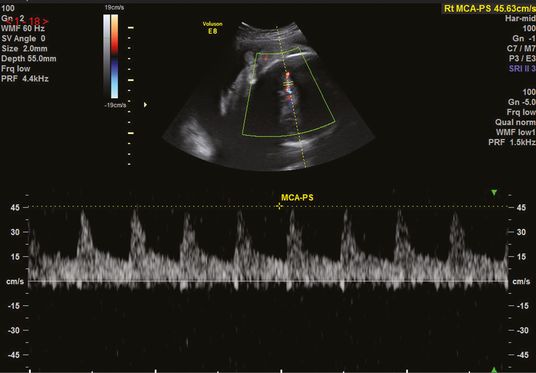
Middle cerebral artery peak systolic (MCA-PS) velocity using manual calipers.
Ductus venosus
The ductus venosus (DV) is a high-velocity small vessel that connects the intra-abdominal umbilical vein to the inferior vena cava just below the diaphragm. This is the most common venous Doppler signal measured. Color Doppler shows aliasing due to the high velocity jet of blood through this narrow vessel, and can be used to identify its position either in the sagittal or transverse plane. Measurement is best done in the saggital plane, which allows alignment with the isthmus of DV without angle correction. DV is a four-phase waveform with a continuous forward flow towards the heart, varying according to different phases in the cardiac cycle. The waveforms consist of the “a” wave, synonymous with atrial contraction of the heart, followed by ventricular systole (“s” wave), then downward slope due to displacement of the mitral or tricuspid valve annulus (“y” descent), and then diastole (“d” wave) (Chapter 21). It is commonly used in the management of SGA/FGR (Chapter 21) to assess fetal cardiac function and in monitoring of twin-to-twin transfusion syndrome (TTTS) (Chapter 25). There may be myocardial impairment, and increased ventricular end-diastolic pressure resulting from an increase in right-ventricular afterload in FGR, which impacts forward flow of blood through the heart. Thus, the a-wave becomes progressively decreased, absent or reversed secondary to the poor cardiac function. A reversed a-wave is a sign of fetal cardiac failure, and is almost always associated with UV pulsations.
In TTTS, the recipient twin suffers from a cardiac failure from vascular overload and DV Doppler is used to monitor diastolic impairment, demonstrated by increased DV PI and a high rate of abnormal end-diastolic flow[11].
Amniotic fluid volume assessment
The amniotic fluid is predominantly produced by the fetus from the second trimester, and thus is used as an indirect indicator of fetal wellbeing. Reduced urine production leading to olighydramnios can be a consequence of reduced renal perfusion due to the redistribution of the fetal cardiac output and shunting blood away from the kidneys to other organs.
Amniotic fluid volume is commonly estimated from a maximum pool depth (MPD), a single measurement of the deepest vertical pocket of liquor, or amniotic fluid index (AFI), the sum of vertical measurement of liquor in four quadrants of the uterus. The pocket of liquor measured needs to be free of umbilical cord loops and fetal parts (Figure 22.4). Diagnosis of oligohydramnios is more common when AFI is used and this leads to more labor inductions and more cesarean deliveries for fetal distress without evidence of improvements in adverse peripartum outcomes, such as admission to NICU, UA pH <7.1 and Apgar score <7 at 5 min[12]. For this reason, most current guidelines recommend that MPD is used for amniotic fluid volume assemment. Although the amount of liquor varies throughout pregnancy, the MPD should be >2 cm throughout pregnancy. Further evaluation of fetal wellbeing is warranted when the MPD is <2 cm as this could be due to prelabor preterm rupture of the membranes, or an early sign of FGR. Similarly, further investigations should be considered when MPD is increased, >10 cm.
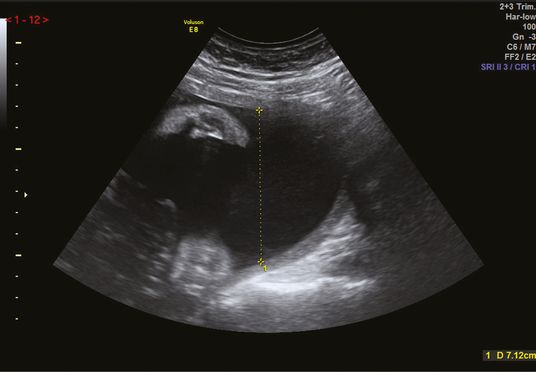
Measurement of the maximum pool depth.
Cardiotocography
Cardiotocography (CTG), a continuous simultaneous recording of FHR and uterine activity was introduced in the 1960s. This test aimed to identify fetuses with acute or chronic hypoxia through the interpretation of FHR patterns, which reflect fetal adaptation to hypoxia. Despite the lack of evidence in reducing perinatal mortality, and morbidity such as hypoxic-ischemic encephalopathy (HIE) and cerebral palsy, this method has been widely used in the antenatal and intrapartum period to assess fetal wellbeing[13]. It is commonly performed from 26 weeks’ gestation, and sometimes even earlier if the fetus was deemed viable. The CTG assessment also forms a component of the formal biophysical profile (discussed below). The CTG is not recommended for low-risk populations, including as an admission test in early labor, as there is no apparent benefit of CTG over intermittent auscultation in reducing perinatal deaths and morbidity[14].
Interpretation of conventional CTG can be subjective, but development of computerized FHR analysis systems has allowed automated evaluation of the CTG through numerical indices, which is more reliable. Computerized CTG (cCTG) analyses are more objective and consistent. Compared to conventional CTG, cCTG has been shown to reduce perinatal mortality when used to monitor fetal wellbeing in high-risk pregnancies[13].
The world famous Dawes–Redman criteria was developed in Oxford University (Oxford, UK) as a computerized system that analyses antenatal CTG recording for fetuses between 26 and 42 weeks’ gestation for 10–60 min, using a set of rules. The first analysis is made after 10 min and assesses a set of criteria including the basal heart rate, accelerations, decelerations, long-term variation, short-term variation (STV) and fetal movements (Table 22.1). The CTG will be classified as ‘reassuring’ when these criteria are met (Table 22.2). If not, the CTG is re-evaluated every 2 min until all the parameters are met, or until 60 min, whichever is sooner. When the CTG has not met the criteria, the reasons are listed to assess if further investigations are needed (Table 22.3).
Special consideration has been given to the STV in the analysis by cCTG. The machine divides each minute of CTG into 16 sections, equivalent to 3.75 s, which contains about 7–10 fetal heart beats, or 6–9 pulse interval (time between two consecutive heart beats). STV is calculated by the average change between these pulse intervals in 3.75 s. STV has been shown to be a reliable indicator of fetal wellbeing, and correlates with acidemia in the fetus. A decreasing STV correlated with earlier deliveries, lower birthweights, lower UA pH at birth, worse acid–base status at birth and worse postnatal outcome. An STV of ≤3 ms (Chapter 21) within 24 h of delivery is associated with higher metabolic acidemia and early neonatal death[15].
| Criteria | Definition |
|---|---|
| High variation | A section of FHR trace in which the long-term variation exceeds a predefined threshold (threshold changes with gestational age) |
| Long-term variation | The average minute range during all or part of a FHR trace |
| Lost beats | The unit of measurement used to describe the size of a deceleration |
| Minute range | The difference in ms between the longest and shortest pulse intervals in 1 min of a FHR trace |
| Pulse interval | The time in ms (milliseconds) between two consecutive fetal heart beats |
| Reactive trace | A fetal heart rate trace that contains at least one episode of high variation |
| Short-term variation | The difference in ms between the mean pulse intervals in consecutive time periods of 1/16th of a minute, averaged over a FHR trace |
| Sinusoidal rhythm | A fetal heart pattern where the trace oscillates smoothly up and down |
FHR, fetal heart rate.
| An episode of high variation (above the 1st centile for gestational age |
| No decelerations (>20 lost beats) |
| Basal heart rate between 116–160 bpm |
| At least one fetal movement or three accelerations |
| No evidence of a sinusoidal FHR rhythm |
| STV >3 ms |
| Either an acceleration or variability in high episodes >10th centile and fetal movements >20 |
| No errors or decelerations at the end of the record |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


