Asepsis and Infection Control
Before the mid-nineteenth century, surgical patients commonly developed postoperative fever followed by purulent drainage from their incisions, overwhelming sepsis, and often death (Mangram and associates, 1999). In the late-nineteenth century, medicine was revolutionized by the observations of Holmes and Semmelweiss, Lister’s publication of antiseptic principles, and acceptance of Pasteur’s germ theory of disease (Larson, 1989). These basic principles remain important today. Although there have been further advances in infection control, including improved operating room ventilation, sterilization methods, barriers, surgical techniques, and availability of antimicrobial prophylaxis, surgical site infections remain a substantial cause of morbidity and mortality among hospitalized patients (Mangram and associates, 1999). This chapter reviews the risk factors for postoperative infection in the obstetric patient. It also reviews the scientific foundation for current recommendations designed to minimize the risk of infection in surgical patients, and the Guidelines for the Prevention of Surgical Site Infections from the Centers for Disease Control and Prevention (CDC). The chapter also includes the CDC’s Standard Precautions, which replaced Universal Precautions (Mangram and associates, 1999).
SURGICAL BARRIERS
In the mid-1980s, concern about blood-borne pathogen exposure began to shift somewhat from the patient to the health care worker. Although the risk of acquisition of blood-borne pathogens following a percutaneous or mucous exposure is low, most surgical barriers are used to protect both the patient and the health care worker. Surgical barriers include gowns, gloves, masks, caps, and shoe covers, as well as surgical drapes. Surgical scrub suits are constructed of a material that is loosely woven for comfort and can reduce exogenous contamination (shedding) by covering as much of the body as possible. Scrub pants and tops are preferable to dresses to prevent particles shed from legs and perineum from contaminating the surgical field (Earl, 1996). Scrub suits serve to prevent transmission and distribution of community-acquired organisms to the operating suite and vice versa. However, controversy exists regarding the laundering of soiled surgical scrubs. Increasingly, hospitals are allowing soiled surgical scrubs to be laundered at home (Belkin, 2001). Although this practice has been opposed by the Association of Operating Room Nurses (AORN), the Centers for Disease Control and Prevention (CDC) points out that there are no data on which to make recommendations on where to launder surgical scrubs and that this remains an “unresolved issue” (Mangram and associates, 1999; AORN, 2000, 1999; Belkin, 2001). Further studies are needed that address the effects of “home laundering” on surgical site infections (SSIs), contamination at home, and the economic impact of such practice.
Surgical gowns, on the other hand, are worn as barriers to microbial transmission during operative procedures, serving to separate sterile from nonsterile areas (Beck, 1981). With the concern of possible exposure to blood-borne pathogens from the patient, barrier efficacy is the most important characteristic to consider when selecting surgical gowns. Penetration of such a barrier is dramatically affected by whether the barrier material is wet or dry. Older standard cloth drapes and gowns were constructed of cotton thread, woven to contain 140 threads per linear inch. Such material is comfortable and when dry is resistant to through transmission of microorganisms even in high humidity (Beck and associates, 1964). However, when such materials become wet with any aqueous liquid, microorganisms are transmitted freely in both directions (Beck and Carlson, 1963; Schwartz and Saunders, 1980; Laufman and associates, 1979). If liquid is wicked from a sterile to a nonsterile site, both sides become contaminated (Beck, 1981). Bacterial transmission through wet material is affected somewhat by the surface tension of the liquid (Laufman and colleagues, 1980). Although experimental transmission rates differ with blood, urine, amniotic fluid, and saline, from a clinical standpoint transmission through standard cloth drapes is instantaneous (Beck, 1981).
These observations led to abandoning the use of standard cloth material as surgical barriers. Today, two types of material are used for gowns and drapes: one, a reusable, tightly woven (280 threads per inch) fabric treated chemically to render it nonwicking, and the second a disposable and impermeable material. Impermeability to moisture is, of course, a basic necessity to any barrier material because the wicking effect tends to transmit bacteria. The materials chosen for these products should be based largely on the level of protection from blood strikethrough needed in each operative procedure, as well as comfort of the product. Gowns constructed with a plastic completely impervious middle layer would prevent strikethrough, but would be intolerable to wear except in the shortest operative procedure (Nichols, 1992). Liquid-proof gowns—those reinforced with films, coatings, or membranes—should be used in the operating room when comprehensive liquid and microbial barrier protection is needed. These gowns provide the optimal protection needed (McCullough, 1993). Leonas and Jinkins (1997) found that higher fabric repellency ratings and smaller pore size generally corresponded with higher barrier proportions. Resistance to microbial penetration in the reusable gown and drapes is lost after 75 launderings, and problems with spot failure with such materials may occur after penetration by towel clips, hemostats, or rubbing. Although modern liquid-impervious materials do not “breathe” well and are less comfortable to the surgeon, their aseptic superiority over older materials is clear.
In addition to drapes that simply cover the skin, adhesive plastic drapes are available that are applied to the skin at the operative site. Two series have shown increased wound infection with the use of adhesive plastic drapes (Cruse and Foord, 1980; Paskini and Lerner, 1969). Other studies found no difference in infection rates when adhesive plastic drapes were used (Chiu and associates, 1993; Cordtz and associates, 1989).
Facemasks are designed to cover the mouth and nose of operating room personnel. Such masks do not form a barrier to microorganisms. Masks are intended to contain and filter droplets of microorganism expelled from the mouth and nasopharynx during talking, sneezing, and coughing (AORN, 1998). OSHA (1991) also requires masks. Careful bacteriologic studies have found no difference in operating room bacterial counts regardless of whether masks are worn (Paskini and Lerner, 1969). Randomized clinical studies also have shown no difference in wound infection rate when masks were eliminated from all operating room personnel except those with upper respiratory infections (Ritter and associates, 1975). Rather than form a barrier to microorganisms, masks redirect them so that they flow out of the sides of the mask (Paskini and Lerner, 1969).
Surgical caps/hoods reduce contamination of the surgical field by organisms from the air and scalp. Various types of hoods rather than caps are being utilized today to cover the hair (Nichols, 1992). According to AORN (1998) recommendations, the head and facial hair, including sideburns and necklines, should be covered.
Although the use of goggles or face shields during surgical procedures probably does not decrease the rate of surgical site infections, they may be of benefit to the health care worker. Some studies found a high risk of contamination by splash during obstetric procedures, particularly for cesarean deliveries (Tichenor and associates, 1994; Sharma and associates, 1997; Popejoy and Fry, 1991). The risk of contamination varies according to the surgical specialty and the role of the members of the surgical team; participants in gynecologic procedures appear to suffer the greatest number of areas of bodily contamination (Hubbard, 1992). Furthermore, Panlilio and associates (1992) found that contamination with blood or amniotic fluid occurred more often in cesarean sections than vaginal deliveries. Protective eye wear or face shields are required by OSHA if splashing or spraying is likely to occur.
Gloves are designed to provide a true aseptic barrier, although no studies exist to document the efficacy of gloves in reducing wound infection. In one series, no wound infections occurred in 141 instances of glove puncture. These authors concluded that organisms escaped in insufficient numbers to be a serious hazard to patients, and speculated that the risk of wound infection would be minimal even without gloves (Dalgleish and Malkovsky, 1988). The importance of gloves, however, in preventing transmission of blood-borne disease to the surgeon is unquestioned (Tuneval, 1987; Dalgleish and Malkovsky, 1988). Unfortunately, gloves are punctured in 11-38 percent of surgical procedures (Dalgleish and Malkovsky, 1988; Serrano and associates, 1991; Cohn and Seifer, 1990). In one series, 62 percent of such punctures were unrecognized, and most occurred in the fingers of the nondominant hand, suggesting that needle handling with fingers may be the most common cause (Serrano and associates, 1991). Double-gloving reduces the risk of blood contamination on the hands of gynecologic surgeons from 38 percent to 2 percent (Cohn and Seifer, 1990). Studies indicate that glove perforations occur with relatively high frequency during pelvic surgery, particularly abdominal procedures, and that double-gloving offers a measure of protection against damage to the inner glove and may prevent subsequent exposure of the surgeon to blood and body fluids (Bennett and Duff, 1991; Jensen and associates, 1997; Kovavisarach and Jaravechson, 1998).
Shoe covers do not affect the incidence of surgical infections and are used mainly to prevent dissemination of body fluids to areas outside the operating room and to protect the health care worker as required by OSHA (1991).
HAND WASHING/SURGICAL SCRUBBING
The goal of the surgical hand scrub is to reduce the number of resident skin bacteria. Resident skin flora will regrow and increase in numbers during the operative procedures (Ehrenkranz, 1992). The accepted practice today is to use an antimicrobial agent to wet the hands and forearms with friction for at least 2 minutes (Larson, 1996; Wheelock and Lookinland, 1997). Antiseptics available for the surgical hand scrub contain alcohol, chlorhexidine, iodine/iodophor, parachlorometaxylenol, or triclosan.
PATIENT PREPARATION
It is well-established that the most important source of potential surgical infection is the patient herself, from bacteria colonizing the skin or, in obstetric surgery, the genital tract (Howard and colleagues, 1964). There is currently no effective method of diminishing colonization of the vagina or uterus other than limiting the number of intrapartum pelvic examinations. However, preoperative preparation of the patient’s skin is of utmost importance (Edlich and associates, 1977). The goal of the preoperative preparation of the patient’s skin is to reduce the number of normal skin flora throughout the operation and thereby decrease the opportunity for a wound infection from this source (Ehrenkranz, 1992).
Hair removal is indicated only if essential for proper performance of the procedure because hair itself does not contribute to infection. Hair removal with a razor can disrupt skin integrity by making small cuts in the skin. Moreover, shaving hair at the operation site the night before surgery significantly increases the rate of wound infection (Polk and associates, 1983; Seropain and Reynolds, 1971; Cruse and Foord, 1980). If hair removal is necessary, depilatory or clipping is preferred to shaving and should be done immediately before surgery (Sherertz and associates, 1996).
A preoperative antiseptic shower or bath decreases skin bacteria colony counts (Hayek and associates, 1987; Byrne and associates, 1990; Garibaldi and associates, 1988; Paulson, 1993; Wihlborg, 1987). Therefore, preoperative bathing of the patient with antiseptics is recommended (Sherertz and associates, 1996).
The skin around the surgical site should be free of soil, debris, and superficial bacteria. The operative site is prepared first by cleaning and then by application of an antimicrobial solution to reduce the body resident flora. The most commonly used preoperative skin preparation agents include iodophors (eg, povidone-iodine), alcohol-containing products, and chlorhexidine gluconate (Mangram and associates, 1999). Chlorhexidine and iodophors have a broad spectrum of activity and are effective in reducing the number of microorganisms on the skin (Wong, 1996). Chlorhexidine has a substantive action after a single application, and, unlike the iodophors, it is not inactivated by blood and serum proteins (Wong, 1996). The incision area commonly is cleansed in a circular motion beginning at the incision site and extending to include the widest foreseeable operative field (Seropain and Reynolds, 1971). Antimicrobial agents used in surgical skin preparation should be applied using sterile supplies and gloves or by using the no-touch technique and proceeding from the incision site to the periphery (AORN, 1996).
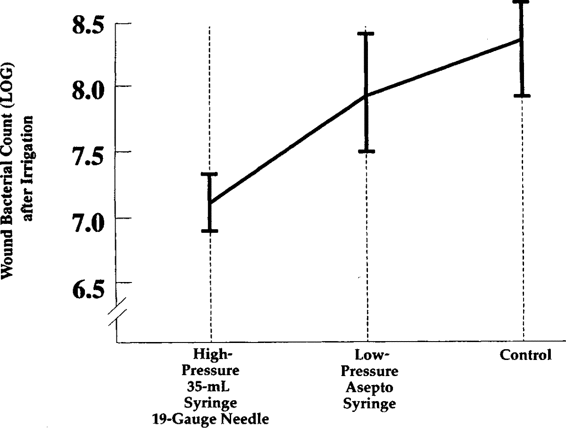
Although intact skin is not damaged by iodophor or chlorhexidine, the introduction of such agents into subcutaneous tissues for irrigation purposes causes tissue damage and actually increases the rate of wound infections (Cruse and Foord, 1980). Irrigation should be carried out only with crystalloid solution. High-pressure irrigation is extremely effective in producing up to a 10-fold decrease in wound bacterial count (Fig. 3-1). Such irrigation may be accomplished using normal saline with a 19-1 gauge needle and 35-cc syringe (Stevenson and associates, 1976).
FIGURE 3-1. Bacterial removal efficiency of fluids delivered by an Asepto syringe or by a 35-mL syringe via a 19-gauge needle. (Reprinted with permission from Stevenson and associates, 1976.)
RISK FACTORS FOR POSTOPERATIVE INFECTION
There are a number of factors that have been associated with an increased risk of postoperative infection (primarily endometritis and wound infections) in obstetric patients (Table 3-1). While some of these factors are modifiable, many are not. They are discussed individually in the section that follows.
TABLE 3-1. Factors that Increase the Risk of Operative Infection in Obstetric Patients
Prolonged labor and/or rupture of membranes
Frequent vaginal examinations
Underlying infection, including intra-amniotic infection and bacterial vaginosis
Underlying disease
Drains
Manual removal of the placenta
PREOPERATIVE STAY
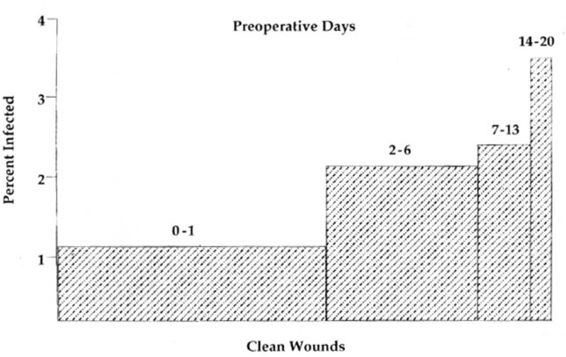
The longer a patient is in the hospital prior to her operation, the greater the risk of wound infection (Fig. 3-2; Cruse and Foord, 1980). These authors found a 1.2 percent infection rate with a 1-day stay, a 2.1 percent infection rate with a 1-week preoperative stay, and 3.4 percent infection rate with a preoperative stay exceeding 2 weeks.
FIGURE 3-2. Relationship of the preoperative hospital stay to wound infection. (Reprinted with permission from Cruse and Foord, 1980.)
DRAINS
Although drains may allow egress of blood and fluid that may serve as a nutrient source for bacteria, they might also allow retrograde access to the wound and operative site. When a drain is needed, a closed system brought out at a different site than the incision is the best choice. In contrast, a relatively high rate of infection is to be expected with the use of Penrose drains exiting from the wound itself. Allaire and colleagues (2000) reported that use of closed suction drainage of the subcutaneous space (with at least 2 cm of subcutaneous fat) in obese women undergoing cesarean delivery may reduce the frequency of wound infections.
CLINICAL INFECTION
Postoperative infections are increased when the patient is clinically infected at the time of surgery, even when the infection is remote from the site of surgery (Polk and colleagues, 1983). In particular, several studies show an increased risk of intra-amniotic infection as well as postoperative endometritis in patients with bacterial vaginosis at the time of labor (Silver, 1989; Watts, 1990; Clark, 1994; and their colleagues). Although there have been no studies to date documenting decreased rates of infection with preoperative treatment of bacterial vaginosis, consideration should be given to examining for and treating this condition prior to delivery when the patient is admitted in labor. Clearly, if bacterial vaginosis is diagnosed prior to delivery, it warrants treatment. In contrast, there is no evidence that routine administration of chlorhexidine vaginal irrigation reduces the incidence of peripartum or postoperative infections (Sweeten, 1997; Rouse, 1997; and their colleagues).
LABOR CHARACTERISTICS
Factors that increase the risk of endometritis following cesarean section delivery include the presence and duration of labor and rupture of the membranes, the number of vaginal examinations, and, perhaps, the use of internal fetal monitors (Casey and Cox, 1997). Because these factors are closely related, it is not clear which factor, if any, is most significant. In a multivariate analysis of 607 laboring women (100 of whom developed endometritis), Newton and colleagues (1990) reported that the presence of “high-virulence organisms” or Mycoplasma hominis in the amniotic fluid was associated with an increased risk of postcesarean endometritis (RR 1.4, P < .01), while the use of prophylactic antibiotics was associated with a decreased risk (RR 0.54, P < .0002). Although significant associations with clinical variables, such as length of labor and use of internal monitoring were found on univariate analysis, these associations were no longer significant on multivariate analysis. The authors concluded that clinical variables were possible “facilitators” but were not predictors of postoperative endometritis.
PLACENTAL REMOVAL
A prospective randomized study of different methods of placenta removal at the time of cesarean section shows that endometritis is significantly more common following manual removal from an exteriorized uterus, as compared to the rate in those who had a spontaneous removal with the uterus in situ, a manual removal and in situ repair, and a spontaneous removal with repair of an exteriorized uterus (Magann and colleagues, 1993). In another prospective randomized study, manual removal of the placenta was associated with an increased rate of postcesarean endometritis, despite the routine use of prophylactic antibiotics (Atkinson and colleagues, 1996). In this study, changing gloves between delivery of the baby and delivery of the placenta did not affect endometritis rates.
OTHER FACTORS
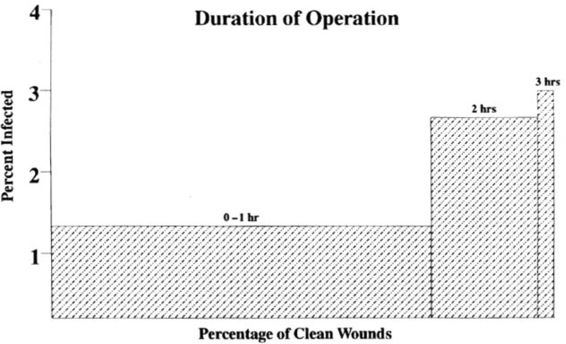
Underlying disease processes, such as obesity, diabetes, malnutrition, uremia, and malignancy may also increase the risk of postoperative infection. Other factors that appear to increase the risk of infection include low socioeconomic status of the patient, operator inexperience and/or skill, a procedure length greater than 1 hour, general anesthesia, and blood loss greater than 800 cc. In cases with a clean wound, infection rates increase directly with operating time; the rate of wound infection roughly doubles with each half hour (Fig. 3-3; Cruse and Foord, 1980; Stevenson and associates, 1976; Public Health Laboratory Service, 1960). Potential explanations for this increase include increasing bacterial inoculum with time, and progressive devitalization of tissue through drying and pressure from retractors. Longer operations also often involve more electrocoagulation and suture placement and a greater likelihood of blood loss and shock (Cruse and Foord, 1980). Clearly, many of these factors are interrelated; however, no study includes a logistic regression analysis to control for the interrelatedness of these risk factors.
FIGURE 3-3. Influence of operative time on wound infection. (Reprinted with permission from Cruse and Foord, 1980.)
RISK FACTORS FOR WOUND INFECTION
In a recent review of 140 women who underwent cesarean delivery, Vermillion and colleagues (2000) report that the thickness of the subcutaneous tissue appears to be a significant risk factor for postoperative wound infections. Devitalization of tissue increases the incidence of wound infections. Wounds that are torn or forced open and injudicious use of cautery increase the risk of wound infection. However,
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree