Fig. 8.1
MRI (axial T2 images) demonstrating right frontal AVM in 2-year-old child. Note dilated vessels in right frontal region, including anterior (ACA) and middle cerebral (MCA) branches, as well as dilated draining veins, both superficial and deep (esp. ultimate drainage to basal veins of Rosenthal). In addition, brain atrophy with sulcal prominence and ex vacuo ventriculomegaly are present
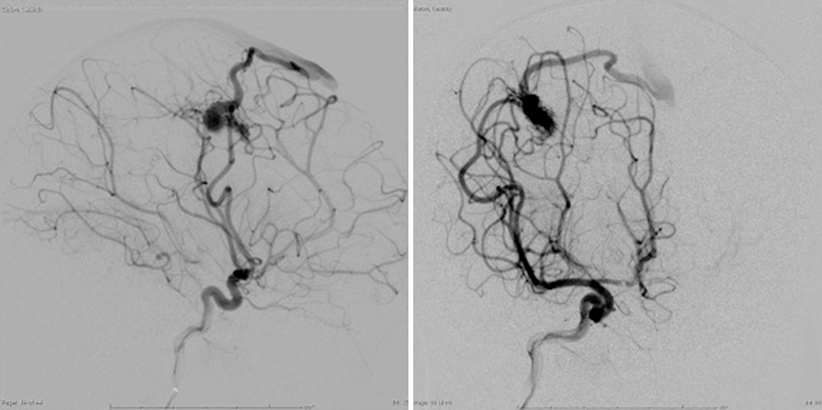
Fig. 8.2
Two projections of left internal carotid artery injection angiogram demonstrating high-flow right frontal AVM in 1-year-old child. Note dilated MCA feeding vessel and early superficial draining vein
Standard preoperative laboratory studies (complete blood count (CBC), clotting times prothrombin time/partial thromboplastin time (PT/PTT), type and cross (T&C) for blood bank, chemistry panel (Chem 7)).
Intervention
Initial therapeutic maneuvers are dependent on the presentation of the child. For the healthy child or for the child who presents with chronic symptoms (seizure, developmental delay), there are often no immediate interventions necessary (with the exception of antiepileptic medication if seizures are present). The following steps are warranted for the child who presents with an intracranial hemorrhage. It is important to note that severity of presentation can vary greatly, and thus treatment must be individually tailored.
Stabilization
Access: Large bore IV (at least 2), arterial line, bladder catheter (airway intubation if unable to protect airway), and nasogastric tube if intubated.
Blood pressure control (labetolol or nipride) with goal of normotension for age.
ICP intracranial pressure control—external ventricular drain if hydrocephalus (NB; avoid overdrainage of cerebrospinal fluid (CSF) to prevent rerupture, often no more than 5 cc at a time), head of bed (HOB) elevated.
Antiepileptic medication if concern for seizure.
Preparation for Definitive Intervention, Nonemergent
As previously discussed, nonemergent management varies greatly depending on presentation. In elective cases, preoperative labs and imaging are needed. For the patient with a hemorrhage but minimal deficits, admission to the intensive care unit for blood pressure control and preoperative imaging studies are the two important interventions. (Emergent management is detailed above.)
Preparation for Definitive Intervention, Emergent
In addition to the steps noted in the stabilization section, the operating room should be notified to prepare for surgery. Equipment should include the operating microscope, multiple suctions, bipolar electrocautery, an array of AVM/aneurysm clips, a craniotome (drill), and a retraction system.
Anesthesia should be consulted and appropriate measures made to ensure that multiple large bore IV access is present and adequate blood products are in the room.
It is helpful to have the microscope draped and clips prepared prior to starting the case if possible, so that quick access can be obtained should unexpected bleeding occur during opening (Fig. 8.3).
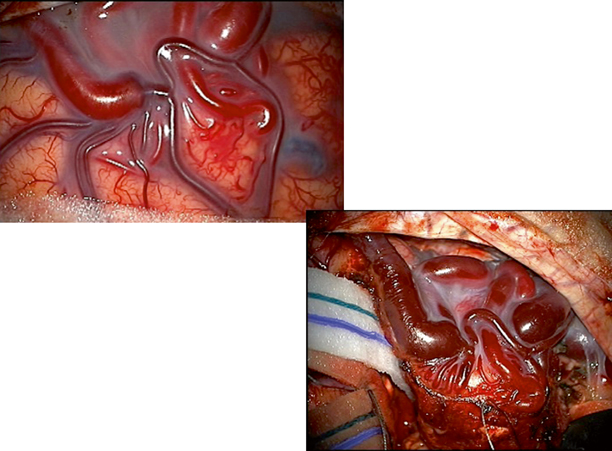
Fig. 8.3
Intraoperative images of superficial cortical AVM. Initial image shows dilate and arterialized draining vein. Subsequent image illustrates method of circumferential dissection around AVM with preservation of draining vein until final portion of case. Note reduction in caliber of draining vein and change to stagnant, deoxygenated venous blood after interruption of arterial supply
Differential Diagnosis
Many patients will be asymptomatic on examination if the lesion is found incidentally. If a spontaneous intracranial intraparenchymal/intraventricular or subarachnoid hemorrhage is found, the following differential diagnosis should be considered:
AVM
Brain tumor
Aneurysm (including mycotic lesions)
Bleeding/clotting disorder
Venous thrombosis
Moyamoya
(Trauma, while not “spontaneous”, should be excluded in recent history)
Diagnosis and Evaluation
Physical Examination
Many patients will be asymptomatic on examination. However, a detailed neurologic examination and history are always important. Attention should be paid to evidence of neurologic dysfunction in the history (see list below).
Findings Suggestive of an Intracranial Lesion
Headache in the early morning hours or awakening patient from sleep
Vomiting
Headache of less than 6 months duration
Confusion or behavioral changes
Abnormal neurologic examination findings
(Positive correlation between number of predictors and risk of surgical lesion)
On examination, findings may be present secondary to (1) local effects (focal weakness, visual changes, etc.), (2) increased intracranial pressure (papilledema, increased head circumference, etc.), or (3) high flow (dilated scalp vessels, bruit on auscultation, cardiac failure).
“Red Flags” on Examination or History
Bradycardia, hypertension, decreased respirations (cushing response)
Dilated pupil, hemparesis (uncal herniation)
Fixed downward gaze (Parinaud’s syndrome)
Lethargy, tense open anterior fontanelle in infants
Ataxia with nausea and vomiting
Sudden onset of a third nerve palsy, including involvement of the pupil (appearing dilated)
Sudden onset of severe headache
Laboratory Data
Standard preoperative laboratory studies (CBC, clotting times (PT/PTT), T&C for blood bank, chemistry panel (Chem 7)).
Imaging Evaluation
CT: If a child presents with a hemorrhage without a clear etiology on initial evaluation, AVM should be considered and repeat imaging in 4–6 weeks with MRI should be performed to evaluate the hemorrhage cavity after the clot has cleared .
AVM typically appears as a heterogeneous area of mixed density with serpiginous areas of enhancement after infusion of contrast material. Cerebral atrophy may sometimes be seen on the affected side. A large malformation or an intracerebral hematoma may distort the normal intracranial anatomy.
MRI is useful for 3D anatomy and identification of chronic ischemia, presumably a result of “steal” phenomena; AVM may be identified on MRI as bright signal of the surrounding brain on FLAIR or T2 images (Fig. 8.1).
The typical MRI appearance is that of a latticework of signal-void spaces, highly contrasted against surrounding cerebral tissue on both T1- and T2-weighted sequences, intermixed with regions of various signal intensities corresponding to blood products in different stages of evolution, and occasionally calcium and hemosiderin [23, 24]. The serpiginous shape of vessels may be distinctive, identified as flow-voids, and relevant anatomy can be well visualized with MR angiography. Susceptibility imaging will sometimes disclose evidence of previous hemorrhage as a dark “bloom” around the nidus [25]. Chronic ischemic changes, presumably a result of “steal” phenomenon or venous hypertension, may be identified on MRI as bright signal of the surrounding brain on FLAIR or T2 images.
Digital subtraction angiography is the definitive investigation. It establishes the nature and extent of the lesion to its blood supply and its venous drainage [26] (Fig. 8.2). Angiography entails bilateral injection of all potential arterial sources of supply to the AVM, both pial and dural (with 15 % of cerebral AVMs receiving some blood supply from ipsilateral or contralateral meningeal arteries [27]): typically at least the internal carotid arteries bilaterally, as well as the ipsilateral external carotid and vertebral arteries. Three-dimensional angiography with computer-generated reconstruction is increasingly employed to depict lesional anatomy. The typical angiographic appearance of an AVM is that of distended, tortuous afferent, and efferent vessels connecting with a tangled vascular mass, through which the circulation time is rapid; i.e., arteriovenous shunting. Other vessels or structures are not displaced unless there is an intracerebral hematoma, which appears as an avascular mass.
A recent analysis of 241 consecutive pediatric patients revealed a 0 % complication rate during the procedure and a 0.4 % post-procedural complication rate. Evaluation should look for:
High-flow vs. low-flow lesions.
Outflow stenoses.
Varices in subarachnoid or ventricular spaces.
Number and location of feeding vessels.
Aneurysms: Flow-related aneurysms are rarely seen in association with AVMs. Often these flow-related aneurysms will spontaneously regress following the reduction in blood flow after treatment of the AVM.
Screening is not justified in the general population. Patients with HHT may be candidates for MRI/A studies of the CNS during childhood to screen for AVMs, as they may be present in 5–10 % of children with HHT.
Nuclear medicine tests
Not usually indicated with AVMs.
Electrodiagnostic tests
Electroencephalography (EEG) may be warranted if the concern for seizure exists.
Neuropsychological tests
Not usually indicated with AVMs, although may be helpful as baseline study in selected children to help with recovery strategies.
Pathology
AVM consist of direct arterial-to-venous connections without intervening capillaries; they occur in the cerebral hemispheres, brainstem, and spinal cord . Functional neural tissue does not reside within the lesion (Fig. 8.4) [1].
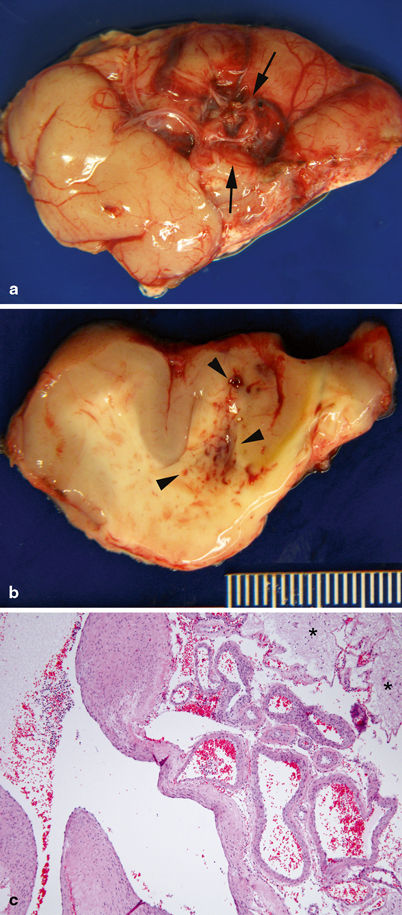
Fig. 8.4
Pathology of central nervous system arteriovenous malformation. a Temporal lobe with arteriovenous malformation (between arrows), characterized by a circumscribed cluster of large tortuous blood vessels. b On cut section, abnormal vessels and petechiae are observed in the white matter (arrow heads). c Large malformed arteries and veins. Large veins have hypertrophic walls with variable thickness, consistent with high-flow, hypertensive changes
Treatment
Goal
To remove the risk of bleeding or growth.
There are no currently accepted medical therapies for the primary treatment of AVM. While adjuvant medical therapy may be helpful (antiepileptic medication for seizure, pain medication for headache, etc.), the obliteration of an AVM is currently achieved by either surgical resection or treatment with radiation.
The treatment goal is complete removal/obliteration of the lesion. Two major modalities are used—surgery or radiation. The decision to operate on an AVM is based on several factors: (1) eloquence of cortical location (speech, motor function, and sensation), (2) pattern of venous drainage, (3) size, (4) associated aneurysms, (5) recent hemorrhage, (6) clinical deterioration, and (7) risk of complication from other modalities of therapy (such as radiation injury to the developing brain) [28, 29]. Several of these factors are combined in the Spetzler-Martin [30] grade that incorporates eloquence of location, pattern of venous drainage, and size and is considered predictive of outcome from surgical management. Spetzler-Martin grade helps to predict surgical risk. If a low grade lesion (1–3) is present, surgery should be considered. Higher-grade lesions (4 and 5) often benefit from multidisciplinary approach and might be considered for radiation therapy.
Spetzler-Martin AVM grading scale | |
|---|---|
Size | |
0–3 cm | 1 |
> 3–6 cm | 2 |
> 6 cm | 3 |
Location | |
Non-eloquent | 0 |
Eloquent | 1 |
Deep venous drainage | |
Not present | 0 |
Present | 1 |
Surgical Therapy
Timing: If a child presents with symptoms of increased ICP then urgent operation may be necessary. If there are worrisome findings on angiography (high-flow lesion with intraventricular varices or aneurysms) then surgery may be planned within the same hospital stay. If not, then a delay of several weeks may help, with delayed reimaging offering better understanding of the anatomy for surgical or radiosurgical planning, as well as an easier surgical approach once the clot has resorbed.
A primary surgical principle for AVM resection is the obliteration of feeding arteries before occlusion of draining veins, as premature closure of outflow can lead to AVM rupture with uncontrolled bleeding.
AVMs are often wedge or cone shaped, and resection can be performed in a circumferential pattern, staying close to—but not entering—the nidus. It is helpful to try to maintain an even depth of resection around the lesion to avoid getting in a “hole” and caution must be taken to minimize retraction on draining vessels during dissection.
Repeated inspection of the surrounding brain for swelling or bleeding can aid the surgeon in preventing complications by early identification of poorly placed retractors or clips (Fig. 8.3).
AVM vessels may coagulate poorly and consideration should be given to clip application or gentle tamponade (if the bleeding is of small volume) if bipolar electrocautery is not working. Every attempt should be made to avoid operating within the nidus itself.
Complications
Bleeding is the most immediate complication of surgery and risks are magnified in smaller children, who have little reserve. The loss of one-quarter of blood volume can induce shock and there may be rapid decompensation in children, which mandates careful monitoring and replacement of blood products by the operative team.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


