(1)
Department of Plastic and Oral Surgery, Boston Children’s Hospital, Harvard Medical School, Boston, MA, USA
Abstract
Arteriovenous malformation is a fast-flow vascular anomaly characterized by the shunting of blood from the arterial to venous circulation. Although it is most commonly observed as an isolated lesion, certain arteriovenous malformations are part of inherited syndromes. This vascular anomaly can cause bleeding, ulceration, congestive heart failure, destruction of structures, and/or disfigurement. Treatment consists of embolization and/or resection. Arteriovenous malformation should be treated in a vascular anomalies center by a multidisciplinary team.
Keywords
Arteriovenous malformationCapillary malformation-arteriovenous malformationEmbolizationPTENIntroduction
An arteriovenous malformation (AVM) is a fast-flow vascular anomaly characterized by the shunting of blood from the arterial to venous circulation . Shunting reduces capillary oxygen delivery to tissues, causing ischemia. AVMs can produce deformity, ulceration, bleeding, congestive heart failure, and/or destruction of vital structures (see Fig. 7.1a). Treatment consists of embolization and/or resection . Certain AVMs are part of inherited syndromes (see Fig. 7.1b, c): (1) capillary malformation-arteriovenous malformation (CM-AVM) , (2) hereditary hemorrhagic telangiectasia (HHT), and (3) PTEN-associated vascular anomaly (PTEN-AVA).
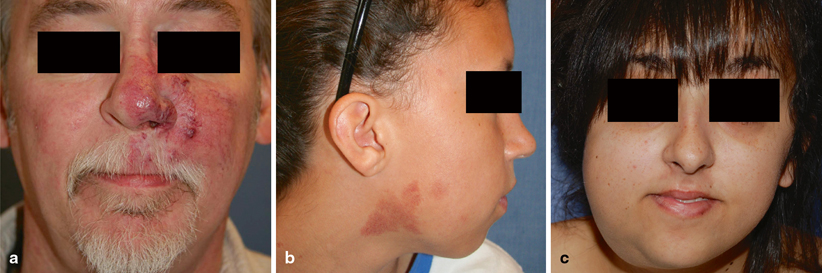
Fig. 7.1
Types of AVM. a Fifty-one-year-old male patient with a Stage II AVM of the left cheek, nose, and orbit, causing epistaxis. b Nine-year-old female patient with capillary CM-AVM (positive for RASA-1 mutation) and fast-flow stains of the right cheek and neck. c Twenty-one-year-old female patient with PTEN-AVA (positive for PTEN mutation) with an enlarging right cheek and submandibular lesion
Key Points
The most common site of extracranial AVM is the head/neck, followed by the limbs, viscera, and trunk [1].
AVM worsens over time, and can be classified according to the Schobinger staging system (see Table 7.1) [2, 3].
Table 7.1
Schobinger staging of AVM
Stage
Clinical Findings
I (Quiescence)
Warm, pink-blue, shunting on Doppler
II (Expansion)
Enlargement, pulsation, thrill, bruit, tortuous veins
III (Destruction)
Dystrophic skin changes, ulceration, bleeding, pain
IV (Decompensation)
Cardiac failure
Despite the high likelihood of recurrence, embolization, and/or resection can palliate an AVM by reducing its size and alleviating pain and bleeding.
AVM should be treated in a vascular anomalies center by a multidisciplinary team.
Biology and Epidemiology
AVM results from abnormal vascular development during embryogenesis. Lack of a capillary bed causes shunting of blood directly from the arterial to venous circulation through a fistula (direct connection of an artery to a vein) or nidus (abnormal channels bridging the feeding artery to the draining veins) [4]. Although the presence of AVM may be problematic, expansion of the lesion is the main cause of morbidity [3].
Pathophysiology
Increasing tissue mass requires neovascularization to support its expansion through angiogenesis (growth of new blood vessels from pre-existing vasculature) [5, 6] or vasculogenesis (de novo formation of new vasculature) [7–9]. Vasculogenesis, rather than angiogenesis, may contribute to the expansion of AVM [10].
Although neovascularization may be a primary stimulus for AVM growth, it might be secondary to ischemia. Ischemia, a potent stimulator of neovascularization, causes enlargement of AVM after proximal arterial ligation or trauma [2, 11, 12]. Alternatively, increased blood flow from arteriovenous shunting may promote vascular endothelial growth factor (VEGF) production and endothelial proliferation [13, 14].
Both males and females have a two-fold risk of progression in puberty; increased circulating hormones during this period may promote AVM expansion [3].
Molecular/Genetic Pathology
CM-AVM is an autosomal dominant condition that results from a loss-of-function mutation in RASA1, which encodes p120RasGAP. This protein inhibits RAS p21 control of cellular proliferation, survival, and differentiation [15].
HHT is due to an alteration in endoglin and activin receptor-like kinase 1 (ALK-1) which affect transforming growth factor-beta (TGF-β) signaling [16, 17].
Incidence and Prevalence
Age Distribution
Sex Predilection
Males and females are affected equally.
Risk Factors
The offspring of patients with CM-AVM or PTEN-AVA have a 50 % risk of inheriting the mutated gene; however, phenotypic heterogeneity is common within families [15, 18, 21].
Progesterone-only oral contraceptives are recommended because estrogen has greater proangiogenic activity than progesterone [1, 22–25].
Pregnant women with Stage I lesions do not have an increased rate of progression, compared to non-pregnant women [3]. However, pregnancy in women with Stage II-IV AVM has not been studied, and thus pregnancy may exacerbate the malformation.
Relationships to Other Disease States, Syndromes
Presentation
Arteriovenous Malformation
Lesions appear pink-red, are warm, have a palpable thrill or bruit, and may be mistaken for a CM or hemangioma [1].
Hand-held Doppler shows fast flow.
Capillary Malformation-Arteriovenous Malformation
Although the CM is rarely problematic, 30 % have associated AVMs that can cause major morbidity: PWS (12 %), extracerebral AVM (11 %), or intracerebral AVM (7 %) [21].
An individual may have as many as 53 CMs, ranging in size from 1 to 15 cm, although 6 % of patients have a solitary lesion [21].
An association between CM-AVM and spinal arteriovenous lesions exists [27].
Five percent of patients have benign or malignant tumors, most commonly involving the nervous system (neurofibroma, optic glioma, vestibular schwannoma) [21].
Patients with PWS should be followed by a cardiologist to monitor signs of congestive heart failure. Orthopedic evaluation is necessary to rule out a leg length discrepancy [21].
PTEN-Associated Vascular Anomaly
Symptoms
Arteriovenous shunting causes ischemia, which can lead to pain, ulceration, bleeding, and congestive heart failure.
AVM also may cause deformity, destruction of tissues, and obstruction of vital structures.
High-pressure shunting of blood can cause venous hemorrhage and rupture of arteries in weakened areas, such as aneurysms.
Arterial bleeding most commonly occurs at skin or mucosal surfaces from erosion into a superficial component of the lesion.
Differential Diagnosis
Capillary malformation (CM)
Congenital hemangioma (CH)
Infantile hemangioma (IH)
Kaposiform hemangioendothelioma (KHE)
Lymphatic malformation (LM)
Pyogenic granuloma (PG)
Venous malformation (VM)
Diagnosis and Evaluation
Physical Examination
Arteriovenous Malformation
Findings
Lesions are usually warm, pink-red, and have a palpable thrill or bruit.
Unlike IH, AVM expands after infancy.
Hand-held Doppler examination showing fast flow excludes slow-flow vascular anomalies (e.g., CM, LM, VM).
Capillary Malformation-Arteriovenous Malformation
Diagnosis is made by history and physical examination. A patient presenting with multiple CMs, especially with a family history of similar lesions, should be evaluated for possible AVMs. Patients are counseled about the autosomal dominant inheritance pattern.
PTEN-Associated Vascular Anomaly
Suspicion of a PTEN-AVA usually is initiated after reviewing the magnetic resonance imaging (MRI) or angiographic study of a patient thought to have an AVM. Vascular anomalies with fast-flow lesions consistent with a PTEN-AVA are evaluated for possible PHTS. PTEN-AVA is an autosomal dominant condition; patients are counseled about the risk of transmitting the gene to their offspring.
Findings
Unlike typical AVM, PTEN-AVA can be multifocal, associated with ectopic fat tissue, and have disproportionate, segmental dilation of the draining veins [4, 18].
Patients with PHTS have macrocephaly (> 97th percentile), and all males have penile freckling [18].
PHTS is associated with mental retardation/autism (19 %), thyroid lesions (31 %), or gastrointestinal polyps (30 %) [18].
Laboratory Data
RASA1 gene testing confirms the diagnosis of CM-AVM. However, not all patients with CM-AVM clinically will have a RASA1 mutation, suggesting that unknown mutations in RASA1 or other genes may result in the same phenotype [21].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


