Background
Although 85% of patients with a complete hydatidiform mole achieve spontaneous remission after a few months, 15% of them will experience gestational trophoblastic neoplasia, which requires chemotherapy. To date, there is no biomarker to predict post–molar gestational trophoblastic neoplasia before the initiation of human chorionic gonadotropin surveillance.
Objective
The purpose of this study was to assess the relationship between the expression of apoptosis markers in the molar villous trophoblasts and the subsequent development of gestational trophoblastic neoplasia after the evacuation of a complete hydatidiform mole.
Study Design
This was a retrospective cohort study of patients with complete hydatidiform mole who were diagnosed, treated, and followed at the Center of Trophoblastic Diseases (Botucatu/São Paulo State and Rio de Janeiro/Rio de Janeiro State, Brazil) from 1995–2014. Patients were divided temporally into derivation (1995–2004) and validation (2005–2014) cohorts. Immunohistochemistry was used to examine tissue expression of the apoptosis inhibitor survivin or the pro–apoptotic enzyme caspase-3. Survivin stains for cytoplasmic and nuclear expression were evaluated independently. Caspase-3 expression was measured as an apoptotic index of positive staining cells over negative staining cells multiplied by 100. Receiver operating characteristic curves were then constructed, and the area under the curve was calculated to test the performance characteristics of the staining to predict the subsequent development of gestational trophoblastic neoplasia.
Results
The final study population comprised 780 patients, with 390 patients in each temporal cohort: 590 patients entered spontaneous remission, and 190 patients experienced post–molar gestational trophoblastic neoplasia. Neither nuclear nor cytoplasmic survivin expression performed well as a predictor of subsequent gestational trophoblastic neoplasia. The caspase-3 apoptotic index was a strong risk factor for subsequent gestational trophoblastic neoplasia development. When the apoptotic index was <4%, the risk of gestational trophoblastic neoplasia had an odds ratio of 35.55 (95% confidence interval, 14.02–90.14; P < .0001) in the derivation cohort and an odds ratio of 25.71 (95% confidence interval, 10.13–65.29; P < .0001) in the validation cohort. However, in both cohorts, the positive predictive value for gestational trophoblastic neoplasia of an apoptotic index <4.0% was modest (49% in the derivation cohort and 41% in the validation cohort); the negative predictive value for gestational trophoblastic neoplasia of an apoptotic index ≥4.0% was high (97% in both cohorts).
Conclusion
The subsequent development of gestational trophoblastic neoplasia after evacuation of complete hydatidiform mole is tied closely to the apoptotic index, which may be a useful biomarker for future prospective studies.
Complete hydatidiform mole (CHM) is a reproductive anomaly that occurs because of a lack of maternal chromosome expression and is characterized by diffuse hydropic villi, marked trophoblastic hyperplasia, and the absence of fetal vessels. Although 85% of patients with a CHM achieve spontaneous remission after a few months, 15% will experience gestational trophoblastic neoplasia (GTN), which requires chemotherapy. Detection of persistence after evacuation of a CHM relies on strict postmolar follow-up evaluation with human chorionic gonadotropin (hCG) surveillance, which is the only marker capable of detecting GTN at an early stage. Efforts to predict postmolar GTN before the initiation of hCG surveillance, such as using the histologic condition of the trophoblastic tumor, the ploidy assessment of DNA by cytometry, cell proliferation markers, and oncogene expression have been unsuccessful. However, a marker that could prognosticate postmolar GTN would have high clinical utility. Patients with CHM at a high risk of experiencing postmolar GTN could be treated with prophylactic chemotherapy, especially in settings in which it is difficult to maintain a rigorous follow-up regimen. Furthermore, the identification of the patients who are at very low risk of persistence could allow shortened surveillance (especially after hCG reaches normal levels) and the reduction of patient anxiety, work absence, and healthcare costs.
Preliminary reports have suggested a relationship between programmed cell death, or apoptosis, and postmolar GTN. Depending on the stimulus, trophoblast apoptosis could be initiated by the intrinsic (mitochondria-dependent) or the extrinsic pathway, mediated by death receptors on the surface of the cell membrane. The intrinsic and extrinsic pathways for trophoblast apoptosis are not mutually exclusive, and both could be activated. These pathways culminate in the action of aspartate-specific proteases called caspases ( c ysteine – asp artic acid – prote ases ), which are responsible for the mediation of cell death by proteolysis at aspartic acid residues. There are also mechanisms for the control of programmed cell death, such as the action of the inhibitor of apoptosis family of proteins, particularly survivin. Survivin is a 16.5 kDA molecule that is expressed during the G2/M phase and is located in the microtubules of mitotic spindles, where it regulates apoptosis by ensuring proper chromosome segregation and cytokinesis. The aim of this study was to evaluate the potential for markers of apoptosis to serve as predictive biomarkers for the risk of GTN after evacuation of a CHM.
Materials and Methods
Design
This was a retrospective cohort study of patients who had been diagnosed with CHM after uterine evacuation and observed and treated at the Trophoblastic Disease Center of São Paulo State University, Botucatu Medical School, and the Rio de Janeiro Trophoblastic Disease Center (33rd Maternity Ward at Santa Casa da Misericórdia) between 1995 and 2014. The research was approved by the Institutional Review Board of the Botucatu Medical School at São Paulo State University (protocol number 497/2007). All the patients previously had given informed consent for participation. Patients were divided into a derivation cohort (comprised of women treated for CHM between 1995 and 2004) and a validation cohort (comprised of women treated for CHM between 2005 and 2014).
Patients
The patients who participated in this study had been diagnosed with CHM, which was confirmed by histopathologic evaluation, and had their molar tissue embedded in paraffin blocks and stored at the Department of Pathology at Botucatu Clinical Hospital at São Paulo State University and the 33rd Maternity Ward of Santa Casa da Misericórdia do Rio de Janeiro. The patients also attended complete postmolar follow-up evaluation at the reference centers for at least 1 year. Patients were excluded if there was not enough histologic material stored for the immunohistochemical study of the molar tissue or there was inadequate material for this study.
Spontaneous remission was defined as 3 consecutive weekly hCG measurements of <5 IU/L. Progression from CHM to GTN was diagnosed by Fédération Internationale de Gynécologie et d’Obstétrique (FIGO) criteria : rising (>10%) hCG levels for 3 consecutive weeks or plateaued for 4 weeks. Patients with a histologic diagnosis of choriocarcinoma or metastases that was detected during postmolar follow-up evaluation, particularly in the lungs and pelvis, were also classified as GTN cases. Before chemotherapy was started, the patients were evaluated for metastatic disease by gynecologic examination, Doppler ultrasonography of the pelvis, and chest radiograph to check for any pulmonary metastases. GTN was staged according to the FIGO 2000 criteria: I, disease confined to the uterus; II, disease extends to the outside of the uterus but is limited to the genital structures; III, disease extends to the lungs, with or without genital tract involvement; and IV, all other metastatic sites.
Prognostic scoring for resistance to chemotherapy followed the FIGO/World Health Organization Prognostic Scoring System. All patients in the current study with postmolar GTN were classified as low risk according to their FIGO 2000 risk factor score and received single-agent chemotherapy (methotrexate or actinomycin-D). Resistance to primary chemotherapy treatment was defined by rising or plateaued hCG levels for at least 3 consecutive weeks. Patients with resistance to single agent chemotherapy received combination chemotherapy (etoposide, methotrexate, actinomycin D/cyclophosphamide and vincristine or etoposide and cisplatin/etoposide, methotrexate, actinomycin D). After chemotherapy, all the patients underwent follow-up evaluation for at least 1 year with monthly hCG surveillance after the first normal hCG value was obtained.
Pathologic condition
The diagnosis of CHM was confirmed by a histologic review of each case by 1 pathologist at each reference center who was not informed of the clinical progression of the disease, using the criteria described by Szulman and Surti : diffuse swelling of chorionic villi with edema, central cistern formation, absence of embryo, and abnormal trophoblast hyperplasia. From 2010 onwards, p57 kip2 immunohistochemistry was used routinely in all cases to distinguish complete from partial mole. For this study, all cases before 2010 were also reviewed, and any case in which there was a question of complete or partial mole was stained for p57 kip2 .
Immunohistochemical study
Histologic sections were made from each paraffin block. Slides were deparaffinized with the use of Xylenes and sequential washes with graded ethanols. Slides were then stained according to an avidin-biotin-peroxidase technique. The following primary antibodies were used in each case: rabbit polyclonal cleaved caspase-3 antibody (Asp175; 1:200 dilution; Cell Signaling Technology, Danvers, MA) and mouse monoclonal survivin antibody (clone 5E8; 1:100 dilution; Neomarkers, Fremont, CA). The histologic sections with the primary antibodies were incubated overnight at 4°C.
The sections were interpreted simultaneously by 2 separate observers who were blinded to the clinical outcome using an optical microscope (Olympus, model BX40; Olympus Corporation, Tokyo, Japan) with ×100 and ×400 magnification. The entire length of each section was observed to select the fields with the most villous trophoblast cells. Histologic sections of tonsil tissue for caspase-3 and gastric cancer for the expression of survivin were used as positive controls. Sections that had been incubated without primary antibodies served as negative controls. Only brown staining in the villous trophoblast cells was considered positive. To measure possible interobserver variation in the interpretation of the immunohistochemical sections, a selection of 30 cases were reviewed independently by 2 pathologists and scored for apoptotic index and nuclear and cytoplasmic survivin staining. The Pearson correlation coefficient between the 2 sets of scores was 0.999 for the apoptotic index and 0.992 for both the nuclear and cytoplasmic survivin staining.
Evaluation of immunoexpression of survivin
Nuclear and cytoplasmic stainings for survivin were scored separately. Intensity and extent of survivin immunoexpression were categorized by a semiquantitative method: negative, weak, moderate, or strong intensity of staining were scored as 0 (negative), 1 (+), 2 (++), and 3 (+++). The extent of staining was either no staining, up to one-third of cells stained, one to two-thirds stained, and more than two-thirds stained, which were scored as 0 (negative), 1 (+), 2 (++), and 3 (+++), respectively. The final survivin immunoexpression score resulted from summing the intensity and extent scores ( Supplemental Figure 1 ).
Evaluation of apoptotic index
Cells were counted with the use of an eyepiece reticule (×400 magnification, which corresponded to 0.25 mm 2 ; Olympus Eyepiece Micrometer; Olympus Corporation, Tokyo, Japan). Cells at the edges of the fields or with unclear nuclei were not counted. On average, 755 ± 80 trophoblastic cells were counted in the spontaneous remission group and 721 ± 87 in the GTN group ( P = .12). The apoptotic index was calculated with a previously described formula, the ratio between the number of caspase-3–positive cells and the total number of cells analyzed multiplied by 100 ( Supplemental Figure 2 ).
Statistical analysis
Parametric statistics were performed with the Student t test, and nonparametric tests were performed with the Mann-Whitney U test. Proportions among groups were compared with the use of a Fisher’s exact test, and linear trends were analyzed with the Mantel-Haenszel chi-square test. Confidence intervals for proportions were calculated according to the modified Wald method. After the patient slides had been scored for the apoptotic index, nuclear survivin expression, and cytoplasmic survivin expression, the results were grouped into strata. The risk of GTN was calculated for each 1% increase in the apoptotic index or each 1-point increase in the survivin immunohistochemistry score. Receiver operating characteristic (ROC) curves were then constructed, and the area under the curve (AUC) was calculated to test the performance characteristics of these classifications with the use of OpenEpi software, version 3.03a. Beta coefficients were calculated with multiple logistic regression analysis with SPSS software (version 21.0; SPSS Inc, Chicago, IL). For each test, a probability value of <.05 was considered statistically significant.
Results
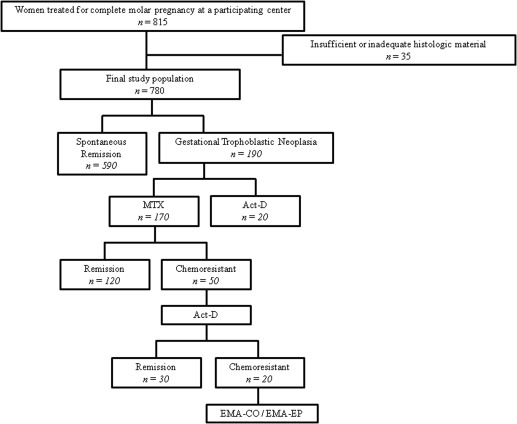
During the study period, 815 patients were treated at the involved centers for CHM ( Figure 1 ). On review, 35 patients were excluded from the study because there was not enough histologic material stored for the immunohistochemical examination or because it was inadequate for this study, which left a final study population of 780 patients. In postmolar follow-up evaluation, 590 patients achieved spontaneous remission, and 190 experienced postmolar GTN. None of the 20 patients who received actinomycin-D as the primary treatment had chemotherapy resistance. Of the 170 patients who received methotrexate, 50 patients (29.4%) experienced resistance and subsequently were treated with actinomycin-D. Twenty of these 50 patients experienced chemoresistance to actinomycin-D and had to be given combination chemotherapy. All patients eventually entered full remission. We considered whether the apoptotic index or survivin expression would be a surrogate biomarker for outcome for patients once the diagnosis of GTN was made. Neither biomarker appeared related to the time to initiate chemotherapy, presence of metastases, chemoresistance, or the need for multiagent chemotherapy ( Supplemental Table 1 ).
The total study population was then divided into 2 temporal cohorts to derive and then validate prognostic scores for the apoptotic index and survivin expression. Patients who were treated at the reference center from 1995–2004 were used as the derivation cohort, and patients who were treated at the reference center from 2005–2014 were used as the validation cohort. Clinical characteristics of the 2 cohorts are presented in Table 1 . Differences between the patients who achieved spontaneous remission vs those who experienced GTN were similar in both cohorts. Consistent with previous reports, patients who went on to experience GTN were significantly older, had larger uteri on presentation, were more likely to have theca lutein cysts, had higher initial hCG levels, and required a longer time to reach remission than patients who had spontaneous remission.
| Characteristic | Spontaneous remission a | Gestational trophoblastic neoplasia b | P value |
|---|---|---|---|
| Derivation cohort, 1995–2004 | |||
| Age, y c | 22 ± 5.8 | 26 ± 6.3 | <.0001 d |
| Gravidity, n c | 0 ± 1.0 | 0 ± 0.8 | 1.0 e |
| Parity, n c | 0 ± 0.9 | 0 ± 0.9 | 1.0 e |
| Gestational age at diagnosis, wk c | 12 ± 4.2 | 13 ± 2.9 | .03 d |
| Uterine size greater than dates, n (%) | 158 (55) | 77 (74.7) | .0006 |
| Presence of theca-lutein cysts, n (%) | 66 (22.9) | 70 (67.9) | <.0001 f |
| Initial human chorionic gonadotropin level (IU/L) | 158978 ± 255998 | 613229 ± 587122 | .001 e |
| Time to remission, wk c | 10 ± 2.9 | 17 ± 4.7 | <.0001 d |
| Validation cohort, 2005–2014 | |||
| Age, y c | 20 ± 4.9 | 25 ± 6.9 | <.0001 d |
| Gravidity, n c | 0 ± 1.0 | 0 ± 0.9 | 1.0 e |
| Parity, n c | 0 ± 0.9 | 0 ± 1.0 | 1.0 e |
| Gestational age at diagnosis, wk c | 13 ± 2.9 | 13 ± 3.4 | 1.0 d |
| Uterine size greater than dates, n (%) | 152 (50.1) | 63 (72.4) | .0003 f |
| Presence of theca-lutein cysts, n (%) | 74 (24.4) | 40 (45.9) | .0002 f |
| Initial human chorionic gonadotropin level (IU/L) | 146978 ± 245891 | 501133 ± 632677 | <0001 e |
| Time to remission, wk c | 10 ± 3.1 | 17 ± 4.9 | <.0001 d |
a Derivation cohort, n = 287; validation cohort, n = 303
b Derivation cohort, n = 103, validation cohort, n = 87
c Data are given as mean ± standard deviation
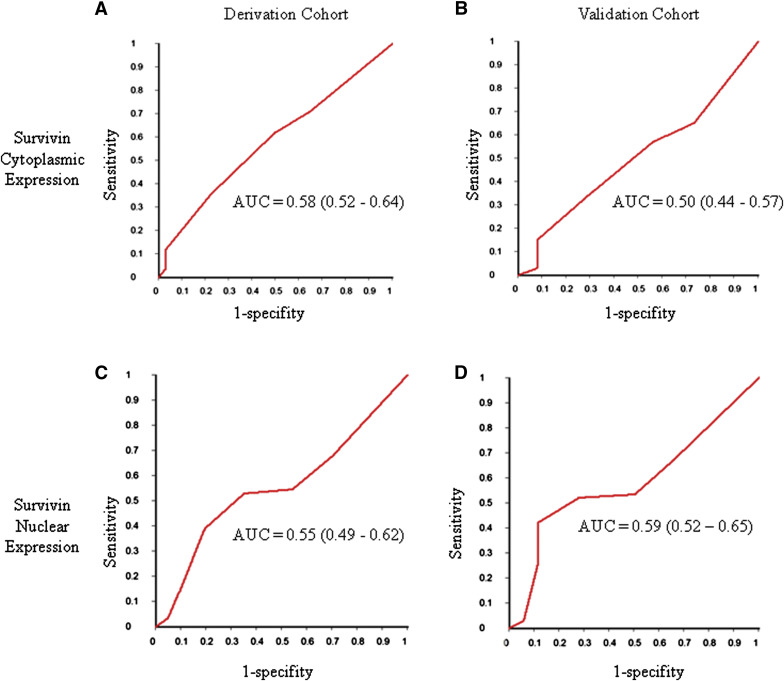
To test the predictive value of apoptosis markers, we began by looking at cytoplasmic and nuclear survivin expression ( Supplemental Table 2 ). In the derivation cohort, immunohistochemistry scores for cytoplasmic survivin tended to be lower in women who had GTN compared with those who achieved spontaneous remission ( P trend = .02). However, the apparent relationship between cytoplasmic survivin expression and GTN was not reproduced in the validation cohort (P trend = .74). In contrast, although the trend for lower nuclear survivin scores among women who had GTN did not quite reach statistical significance in the derivation cohort ( P trend = .08), there appeared to be a stronger association in the validation cohort ( P trend = .005). However, Figure 2 shows that, when ROC curves were constructed for cytoplasmic and nuclear survivin expression for both cohorts, neither biomarker had strong performance characteristics; both cytoplasmic and nuclear survivin expression were only slightly better than chance alone.
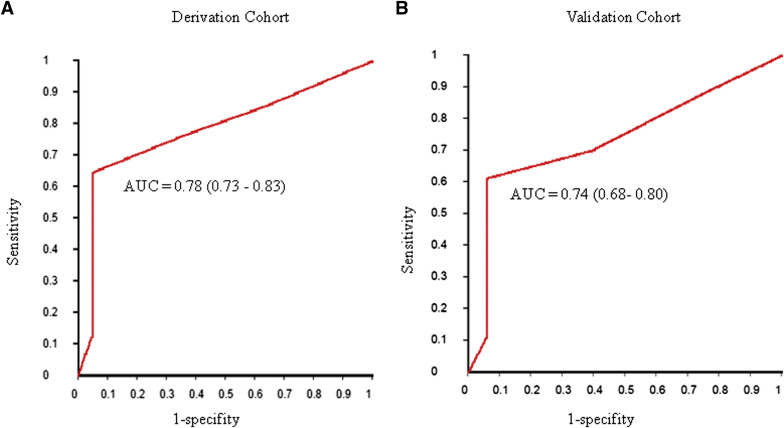
Next, we looked at the relationship between the apoptotic index and the subsequent development of GTN ( Supplemental Table 3 ). Apoptotic indices tended to be low in women who experienced GTN and high in women who achieved spontaneous remission ( P trend <.0001 in both cohorts). In both the derivation (AUC = 0.78; 95% confidence interval [CI], 0.73–0.83) and the validation cohorts (AUC = 0.74; 95% CI, 0.68–0.80), the apoptotic index performed relatively well in the prediction of the subsequent development of GTN ( Figure 3 ). Based on the ROC curves, the optimum cut-off for the prediction of GTN appeared to be an apoptotic index of 4.0% ( Table 2 ). In the derivation cohort, for an apoptotic index <4.0%, the risk of GTN was 98 of 200 (0.49; 95% CI, 0.42–0.56), compared with a risk of 5 of 190 (0.03; 95% CI, 0.01–0.06) for an apoptotic index ≥4.0%, for an odds ratio of 35.55 (95% CI, 14.02–90.14; P < .0001). This was reproduced in the validation cohort, where the risk of GTN was 82 of 200 (0.41; 95% CI, 0.34–0.48) for an apoptotic index <4.0%, and 5 of 190 (0.03; 95% CI, 0.01–0.06) for an apoptotic index ≥4.0%, for an odds ratio of 25.71 (95% CI, 10.13–65.29; P < .0001). Although in both cohorts, the positive predictive value for GTN of an apoptotic index <4.0% was modest (49% in the derivation cohort and 41% in the validation cohort), the negative predictive value for GTN of an apoptotic index ≥4.0% was high (97% in both cohorts).