Antipyretics
John T. Wilson
Victoria Tutag Lehr
Erika Crane
Margaret Ann Springer
Introduction
Fever has been recognized since antiquity and variously attributed to a myriad of causes from excessive yellow bile to demonic possession. This complex entity has been poorly understood, inconsistently diagnosed, and treated. Fever, or perceived fever, continues to be one of the most common reasons for a child’s presentation for medical care (1). However, opinions vary widely among clinicians, parents, and patients regarding the exact definition of fever, its physiology, function, and management. Variability exists in the methods, instruments, and body sites for appropriate assessment of body temperature.
This chapter uses the definition of fever established in 1987 by the International Union of Physiological Sciences Thermal Commission, namely, “a state of elevated core temperature, which is often, but not necessarily, part of the defensive responses of multicellular organisms (host) to the invasion of live (microorganisms) or inanimate matter recognized as pathogenic or alien by the host” (2). Fever as a component of the febrile response involves a cytokine-mediated rise in core temperature, generation of acute-phase reactants, and activation of numerous physiologic, endocrinologic, and immunologic systems (2). It is important to distinguish fever from hyperthermia, an unregulated rise in body temperature that does not involve pyrogenic cytokines and is unresponsive to antipyretics (2). The clinical dilemma “to treat or not to treat,” and the clinical pharmacology of antipyretic drugs for use in pediatric patients will be discussed.
Physiology of Fever
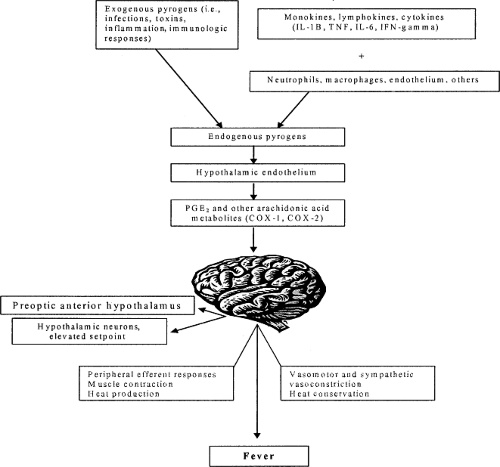
Understanding fever requires a review of the body’s thermoregulatory system, a complex neural network extending from the hypothalamus and limbic system through the lower brain stem and reticular formation into the spinal cord and sympathetic ganglia. Thermosensitive neurons in the preoptic region of the rostral hypothalamus are important in regulation of the thermoregulatory system, which maintains the body’s temperature within a narrow range via a central entity termed the “set point.” No longer regarded as a single temperature perceived at a single anatomic site, the set point is thought to be a range of temperatures perceived in multiple areas of the hypothalamus. Deviations from the set point can provoke multiple thermoregulatory responses (2). When the preoptic temperature rises above its set point, based on circulation-borne neuronal signals from thermosensors throughout the skin and core areas of the body, physiologic heat-loss responses are triggered. If the temperature falls below the set point, heat-retention and heat-production responses are activated (2,3).
During activities such as exercise, excess heat is generated by normal biochemical processes; the thermoregulatory response is heat loss until body temperature returns within normal range. When the body temperature falls below the normal range, heat conservation processes ensue. In response, pyrogens alter the function of the hypothalamic neurons and raise the set point. The febrile response is stimulated with endogenous mediators of fever activating a series of responses to decrease heat loss and increase heat production. This contrasting phenomenon that produces fever is clinically recognized as “a pyrogen-mediated rise in body temperature above the normal range” (2). Viewed in this context, fever is regarded by many to be an adaptive response, potentially beneficial to the patient.
Cytokines are among the more important pyrogens. These pleiotropic, intensely powerful proteins function singly or in groups to convey information from cell to cell within a complex network. The febrile response is mediated by exogenous and endogenous pyrogens. Exogenous pyrogens are of microbial origin inducing host cells, especially macrophages, to produce endogenous pyrogens. The most common endogenous pyrogenic cytokines are interleukin-1 (IL-1), tumor necrosis factor-α (TNF-α), IL-6, and interferon-#979; (IFN-#979;). These cytokines interact with receptors in the anterior hypothalamus to activate phospholipase A2, which in turn liberates plasma arachidonic acid as a substrate for the production of prostaglandin E2 (PGE2), catalyzed by cyclooxygenase (COX). Prostaglandin E2 in turn resets the hypothalamic set point and produces fever (see Fig. 41.1). Antipyretic drugs interrupt this pathway producing their pharmacologic effect (2).
Pitfalls of Site-Dependent Measurement of Fever
Diagnosis of fever in the clinical setting requires precise measurement of body temperature and characterization of that measurement as elevated. This is problematic in pediatric practice because there is no universal definition of “normal” body temperature for children of various ages. Important unresolved issues exist concerning age-related fluctuations in body temperature. In addition, there is lack of consensus on the optimal body site for measuring body temperature or the most appropriate thermometer that should used for this measurement (5). A 1995 survey demonstrated wide variation in perceptions of normal body temperature among physicians, medical students, and graduate students (6). There is long-standing disagreement on the merits of various anatomic sites (rectum, mouth, axilla, tympanic membrane, temporal artery) as accurate reflections of body temperature (5,6). Many types of thermometers are marketed, ranging from oral, rectal, and axillary, to infrared thermometers that measure the arterial blood temperature in the tympanic membrane (7). A recent innovation in thermometry measures the temperature of the temporal artery (8). Supralingual digital pacifier thermometers may provide a convenient method of measuring temperature in children younger than 2 years (9,10). The sensitivity and specificity of the pacifier thermometer has been compared with tympanic and glass mercury thermometers in children younger than 2 years (9). In this small study (n = 81), temperature measurements were obtained at supralingual, tympanic, and rectal sites using the mercury thermometer as the standard. Overall, the pacifier thermometer was shown to provide temperature measurements similar to the tympanic device, providing an alternative assessment method for small children. Another smaller investigation (n = 25) comparing pacifier and rectal temperatures in children aged 7 days to 24 months in a pediatric hospital reported
high correlations between rectal and pacifier temperature measurements (10). These data support the use of this type of device as an alternative method for measuring temperature in young children at home, although the relatively high cost of the device, US $15.00, and long duration (6 minutes) required to obtain a temperature measurement may be a barrier to use in clinical settings and for some parents.
high correlations between rectal and pacifier temperature measurements (10). These data support the use of this type of device as an alternative method for measuring temperature in young children at home, although the relatively high cost of the device, US $15.00, and long duration (6 minutes) required to obtain a temperature measurement may be a barrier to use in clinical settings and for some parents.
Infrared tympanic thermometers are commonly used for children in emergency departments and clinics secondary to their ease of application in the external auditory canal and rapid, accurate measurement (7,11,12). Tympanic thermometry has been demonstrated to be more accurate than using an electronic axillary thermometer for measuring temperature in febrile and afebrile infants (n = 106) in a pediatric emergency department (12). Body temperature was measured using infrared tympanic thermometry, an electronic axillary thermometer and a standard rectal thermometer. For febrile and afebrile infants, tympanic temperature measurements correlated more closely with rectal temperatures than the axillary temperatures. There was also a lower mean difference between the tympanic and rectal temperatures compared with the tympanic and axillary temperatures. This is not unexpected as core temperature can be obtained by direct contact with the tympanic membrane (7). However, the process of obtaining an electronic tympanic temperature was demonstrated to be five times faster than using the axillary method.
When considering thermometers for pediatric use, the optimal device would allow accurate, fast, safe, and convenient measurement of temperature at a reasonable cost. In 1999, the US Environmental Protection Agency recommended that mercury no longer be used for thermometers (13). The agency advises that these devices be replaced with mercury-free alternatives. Children are more sensitive to the effects of mercury toxicity compared with adults; therefore, a broken mercury thermometer is a potential hazard (13). Many hospitals and health care systems have converted to mercury-free medical equipment.
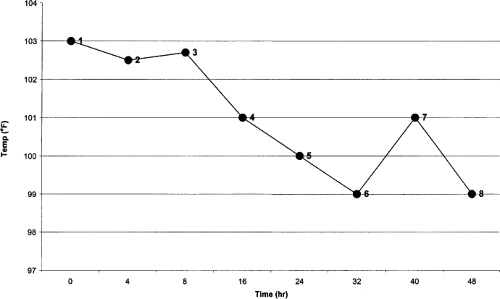
An accepted definition of fever in children is a rectal temperature greater than or equal to 38#65518;C or 100.4#65518;F (14). All too frequently, however, little attention is given to consistency of site or technique for measurement, or to observer error. For example, the rectal site is seldom used in children older than 1 year. Figure 41.2 illustrates some of the many difficulties of temperature measurement and interpretation in a 1-year-old child admitted for suspected sepsis.
These site differences are consistent with the work of Brown and Wilson (5), who found respective mean differences (in degrees Fahrenheit) from rectal temperature of 1.31, 2.23, 4.28, and 5.75 for oral, axillary, abdominal skin, and forehead skin, respectively. A probability nomogram gave the best predictive power for likelihood of differences between sites. These investigators (14) and others (15) also proposed area under-the-curve estimation as a more accurate means of documenting temperature height and fever duration, noting that this method is especially useful when site differences and antipyretic drug efficacy are compared (5,14,16).
Obviously, the data in Figure 41.2 are misleading; using such data without appropriate explanation and interpretation could hinder effective clinical practice. For temperatures
1 to 3, a fever is documented. Because the temperature is lower at temperatures 4 to 6, it is assumed that the prescribed antibiotic regimen was therapeutically appropriate, when the real reason for the perceived drop in temperature is the change of measurement site. Until temperature 7 is measured (rectally), persistence of the fever is not evident. The temperature measured at 8 is axillary, and is a reflection of the site, rather than an actual temperature spike. Alternatively, caregivers could have changed antibiotic treatment at 7 and viewed point 8 (erroneously) as defervescence rather than as a site-selection difference. Similar problems could result from failure to consider the effect of the use of different measuring devices (thermometers, electronic thermocouples, infrared ear devices). It is difficult to discuss fever and its management if methods for temperature measurement are inadequately documented in clinical practice and in the literature.
1 to 3, a fever is documented. Because the temperature is lower at temperatures 4 to 6, it is assumed that the prescribed antibiotic regimen was therapeutically appropriate, when the real reason for the perceived drop in temperature is the change of measurement site. Until temperature 7 is measured (rectally), persistence of the fever is not evident. The temperature measured at 8 is axillary, and is a reflection of the site, rather than an actual temperature spike. Alternatively, caregivers could have changed antibiotic treatment at 7 and viewed point 8 (erroneously) as defervescence rather than as a site-selection difference. Similar problems could result from failure to consider the effect of the use of different measuring devices (thermometers, electronic thermocouples, infrared ear devices). It is difficult to discuss fever and its management if methods for temperature measurement are inadequately documented in clinical practice and in the literature.
Perceptions of Fever: Misinformation and Myth
Another complicating factor in the diagnosis and management of fever is “fever phobia,” first described by Dr. Barton Schmitt in 1980. His study revealed that parents often view fever as a disease rather than a sign or symptom. Schmitt found that many caregivers believed that fever could cause serious side effects, such as brain damage (17). As Crocetti and colleagues reported in 2001, misconceptions about fever and its role in disease process persist in the new millennium (18). In a study of 340 caregivers in an urban setting, 91% reported that they believed that fever could cause brain damage and death, and that a temperature could rise to 110#65518;F if left untreated (17). Both studies documented numerous inaccurate beliefs about what constituted fever, how often it should be checked, and how often it should be treated (17,18). Fever phobia still causes parental anxiety and may lead to excessive fever monitoring (sometimes at intervals of <1 hour), overzealous antipyretic treatment, and inappropriate use of other fever-reducing practices, such as sponging (17). More recently, Crocetti’s study was replicated in the United Kingdom with similar results in terms of parental fears, level of parental anxiety, aggressive measurement, and treatment of childhood fever (19).
Factors such as education level, socioeconomic status, and insurance coverage may also influence how a parent perceives and responds to childhood fever (20). Similarly, recent research has focused on similarities and differences in fever perception and management in various ethnic and cultural groups (20). Specifically, the parents’ attributed cause of fever as well as use of complementary or alternative therapies to treat fever may vary greatly between ethnic groups (21,22). It is incumbent upon the health care provider to ask parents about their ethnic or cultural practices for handling fever and counsel as appropriate.
Caregivers who do not understand fever physiology may be overanxious to treat their children. However, many health care providers do not define “high” fever or explain its function in the body’s defensive response (23). Health care providers who fail to educate themselves, their patients, patient’s parents, and caregivers about the nature of fever, its significance, and its appropriate treatment may perpetuate fever phobia and its consequences (18,24). One consequence of fever phobia may be that about 50% of parents make dosing errors when administering acetaminophen (APAP) and ibuprofen (IBU) for fever to their infants and children; more than one-half of the caregivers surveyed in a study administered inappropriate doses of both drugs (25).
Controversies of Management
Provocative articles published since 1992 primarily address management of the febrile child from the standpoint of extent of illness, etiologic evaluation, and treatment (26,27,28,29,30,31,32,33). A guideline that emerged was stratification based on fever, as well as age, white blood cell count, and suspected site of infection. Advocated evidence-based approaches neglected prior or concomitant use of antipyretics to alter temperatures observed. Although recent or partial treatment with an antibiotic may modify a pyretic response, less recognized or cited is the role of antipyretics. This issue is exacerbated by approximately 50% of parents giving inaccurate information about use of these drugs (34). Overall evaluation and management of fever must consider confounding by the ubiquitously available APAP or IBU. Indeed, the temperature profile of Figure 41.2 could have been produced by an unrecognized or unreported administration of antipyretics given by nurse or parent on a pro re nata basis, removing a valuable index of disease severity. In children, a delay in initiation of antibiotic therapy occurred in those given an antipyretic (35).
A defensive or beneficial role of fever argues against the use of antipyretic drugs. This issue was summarized recently by Mackowiak and others (36,37,38). Admonitions in support of treatment are often rooted in fever phobia (39) or the comfort of caregivers who believe that fever per se is noxious. Examples abound that it may not be (36), even at different phylogenetic levels. Elevation of body temperature has enhanced a resistance to some viruses and bacteria in mammals. In humans, a positive correlation between temperature and survival was noted in those with bacterial infection. The adaptive response of fever is suggested by prolongation of viral illnesses in those receiving an antipyretic. Consistent with a beneficial effect is that pyrogenic cytokines were found to have immune-potentiating effects or to enhance resistance. On the other hand, they may enhance physiologic abnormalities associated with serious infection such as a gram-negative sepsis or that provoked artificially by lipopolysaccharide. Brandts and coworkers (40) found evidence for prolongation of APAP-treated-children’s response to malaria. APAP was associated with an increased period for lesion crusting in children with varicella (41). In our opinion, altered cytokine release via modulation of fever has of yet an unknown relation to organism, site involved, and severity of infection. More data are needed for antipyretic drug treatment guidelines on this basis alone.
Another point to consider is the danger from a high fever (36,42), which apparently has not been demonstrated at or rarely above 41#65518;C. Of concern, however, are special populations with cardiac or pulmonary disorders
where antipyretic treatment may lessen metabolic demands. Mackowiak (36) asserted that this effect on the risk/benefit ratio has not been substantiated, especially from the standpoint of effects on coronary blood flow and loss of fluid with sweating (summarized in reference 43). The recurrence of febrile seizures does not appear to be influenced by antipyretic drugs (44,45,46,47,48).
where antipyretic treatment may lessen metabolic demands. Mackowiak (36) asserted that this effect on the risk/benefit ratio has not been substantiated, especially from the standpoint of effects on coronary blood flow and loss of fluid with sweating (summarized in reference 43). The recurrence of febrile seizures does not appear to be influenced by antipyretic drugs (44,45,46,47,48).
Another area of controversy is the use of antipyretics to prevent postvaccination febrile responses in children. Currently, the American Academy of Pediatrics (AAP) and the Advisory Committee on Immunization Practices recommend prophylactic postvaccination antipyretic therapy only in children at a higher risk for seizures than the general population. Despite this recommendation, the incidence of postimmunization use of APAP and IBU in pediatric practices is 89% and 56%, respectively (49). A recent meta-analysis found that neither APAP nor IBU reduced the incidence in postimmunization fever in children after administration of the DTaP vaccine (50). This finding was confirmed in a 2008 randomized controlled trial (51). Similarly, rates of postimmunization pain and local skin reactions were also not affected by the use of prophylactic antipyretics (49,52).
Relief of discomfort and controversial effects on the host immune response may be due to anti-inflammatory and analgesic rather than (or in addition to) antipyretic properties of IBU and APAP. These agents effectively relieve pain, arthralgias, and myalgias that often accompany fever producing illnesses (43). Notable is the beneficial effect found with IBU on mortality of hypothermic patients with sepsis (53). However, in another report the multiple effects of IBU, including a lowering of body core temperature, did not affect organ failure or mortality in patients with sepsis (54). Combined relief of symptoms affects drug selection. For fever alone, and not to obscure signs of an underlying inflammatory illness, then APAP is reasonable combined with a opioid (e.g., codeine) if moderate pain relief is needed. IBU provides relief of fever, pain, and inflammation at appropriate doses. Preservation of fever as a sign of disease in those children with pain supports selection of an opioid analgesic without antipyretic properties. Understanding the pathophysiology of fever, pain, and inflammation will likely lead to the development of selective-action drugs. Until then, one must realize that more is being affected, perhaps deleteriously, than fever. The effect of antipyretic drug action on children must be considered if the short-term solution of antipyresis will be exchanged for adverse outcome-altered host response or morbidity as in the case of Reye syndrome associated with aspirin use (55,56,57).
Modulation of fever by antipyretic drug use failed to be of diagnostic significance (58) for discerning disease severity or its bacteremic nature. This failure may be partly due to the nonlinear pharmacokinetics of these drugs (16,59). Some suggest that nonsteroidal anti-inflammatory drugs (NSAIDs) (naproxen, indomethacin, diclofenac) may be more effective for fever from cancer than from infection (60,61,62). We are unaware of pediatric studies that have shown this distinction, although it would be important for drug selection.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree