Anatomy, Incisions, and Closures
Factors impacting the surgeon’s choice of abdominal incision and closure for the obstetric patient include the indications for the operation, the urgency for operative intervention, associated preoperative conditions, and the presence of previous abdominal scars. Incisions should be selected with the objectives of an appropriately rapid entry, adequate exposure, and closure that reduces the likelihood of infection or dehiscence. This chapter reviews important factors affecting wound integrity to prepare the surgeon for choosing the incision most appropriate to each clinical situation.
ANTERIOR ABDOMINAL WALL ANATOMY
An intelligent choice of incision depends on a thorough understanding of the anatomy of the abdominal wall. Not only does this permit the surgeon to avoid injury to nerves and blood vessels, but it also minimizes the chance of dehiscence and promotes effective healing. The qualities of the abdominal wall tissues, including the direction of muscle contractility and lines of skin and fascial elasticity, affect healing and the resultant scar appearance and strength. The distribution of the vasculature and innervation of the abdominal wall also impact postoperative healing and function. Therefore, important anatomic parameters for the surgeon to consider include the overlying skin, the abdominal wall muscles, their fascial sheathes and aponeuroses, and the abdominal wall vasculature.
The boundaries of the abdominal wall are the costal margins of the seventh through tenth ribs and xiphoid process superiorly, the inguinal ligaments, pubic crests, and symphysis pubis inferiorly, and the iliac crests laterally (Fig. 4-1). The skin of the abdominal wall has lines of cleavage, referred to as Langer’s lines after the Austrian physician that described them in 1861. These cleavage lines run in a nearly transverse direction. Incisions made parallel to Langer’s lines heal with finer scars and tend to be more cosmetic.
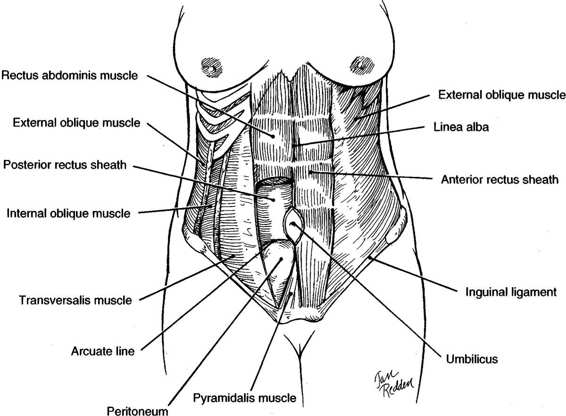
FIGURE 4-1. Anatomy of the anterior abdominal wall showing major muscle groups.
MUSCLES AND FASCIAL SHEATHES
The anterior abdominal wall has two groups of muscles, one with fibers running transversely and one with fibers running vertically (Fig. 4-1). The transverse or flat muscles are the deepest group of muscles and include the external oblique muscles, the internal oblique muscles, and the transverse abdominis muscles. Superficial to the transverse group is the vertical group composed of the rectus abdominis muscles and pyramidalis muscles.
The transverse muscle groups and their fascial sheathes provide much of the strength of the abdominal wall. The most superficial are the paired external oblique muscles. Superiorly, their fibers run transversely, while inferiorly, they assume an oblique downward course. Posterior to the external obliques lie the paired, fan-like internal oblique muscles. The midportion of the muscles run in an upward oblique course and give rise to the aponeurosis of the internal oblique muscles. Beneath the internal obliques are the transverse abdominis muscles, which have a truly transverse course. The final layers are the transversalis fascia, preperitoneal fat, and, lastly, the peritoneum. These three muscle pairs fuse in the midline at the linea alba by means of their fascial aponeuroses.
The major support of the central portion of the abdominal wall comes from the paired rectus abdominis muscles, which lie on either side of the linea alba. These muscles arise from the costal margins of the fifth through eighth ribs and insert into the superior pubic rami (Fig. 4-1). The rectus abdominis muscles have three to four fibrous insertions, the linea transversae. One is at the level of the umbilicus; the other two are usually halfway between the umbilicus and the insertions. Importantly, the fibrous insertions are tightly adherent to the anterior rectus sheath, which limits its retraction when cut. The rectus muscles with their thin investing fascia are muscles of locomotion and posture and are not essential to the integrity of the anterior abdominal wall (Daversa and Landers, 1961). The other vertical muscle groups are the paired pyramidalis muscles. These triangular muscles, which lie anterior to the rectus abdominis muscles, arise from the superior pubic rami and insert into the lower linea alba. The midportion of this muscle usually has an avascular raphe, which can easily be incised for adequate exposure of the space of Retzius. While they provide some support to the lower abdominal wall, their function is minor and their surgical preservation is not essential.
A portion of the external oblique muscle aponeurosis gives rise to a broad fibrous sheath that courses medially and anterior to the rectus muscle. The internal oblique muscle aponeurosis splits at the lateral border of the rectus abdominis muscle and forms a sheath around the rectus muscle, rejoining medial to the rectus muscle to form the linea alba (Fig. 4-1). The transverse abdominis has a truly transverse course. Above the midway point between the umbilicus and pubis, the aponeurosis of this muscle passes behind the rectus muscles and contributes to the posterior rectus sheath. Below this point, the aponeurosis passes anterior to the rectus muscles, contributing to the anterior rectus sheath (Fig. 4-1). The lower limit of the upper portion of the transverse aponeurosis, which is situated posterior to the rectus muscle, forms a crescentic border, the arcuate line. Below the arcuate line, near the level of the anterior superior iliac spines, the posterior rectus sheath is absent. If proper closure methods are not used, hernias or fascial dehiscence are more likely to occur in this area.
ABDOMINAL WALL VASCULATURE
The anterior abdominal wall has an excellent blood supply with branches of three major arteries, including the external iliac, femoral, and anterior thoracic arteries. Because of their rich anastomoses, vascular deficiency is usually not a complication of abdominal wall surgery. The medial abdominal wall receives blood from the epigastric arteries, while the musculophrenic and superficial and deep circumflex iliac arteries (Fig. 4-2) supply the lateral wall.
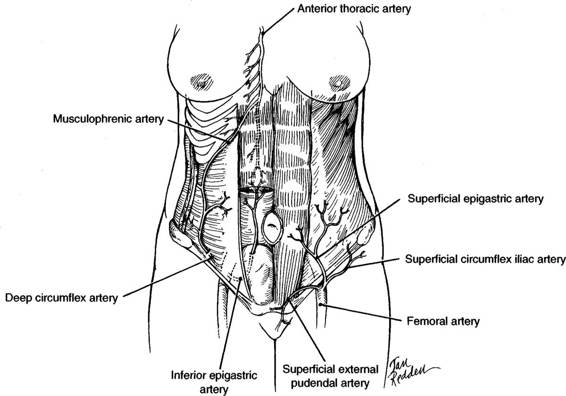
FIGURE 4-2. The major arterial blood supply of the anterior abdominal wall. The four major vessels—two medial and two lateral—contribute to the rich anastomoses. The superficial epigastric artery serves the superficial tissues of the lower abdominal wall.
The superior epigastric artery, a branch of the anterior thoracic artery, enters the sheath of the rectus abdominis muscle from behind the seventh costal cartilage and descends posterior to the rectus muscle. Above the umbilicus, the superior epigastric artery tends to lie posterior to the midportion of the rectus muscle. The inferior epigastric artery arises from the external iliac artery near the midinguinal point and courses in a cephalad direction along the posterior lateral portion of the rectus muscle. Because the inferior epigastric artery course runs from lateral toward the midline (Fig. 4-2), a low transverse incision is unlikely to damage these vessels. The superior epigastric artery anastomoses with the inferior epigastric artery; and these vessels supply multiple branches into the substance of the rectus muscle. The veins lie in close approximation to the main trunks of the epigastric arteries.
The musculophrenic artery runs along the costal margin behind the cartilages. It has anastomoses with the deep circumflex iliac artery, which originates from the external iliac artery at about the same level as the inferior epigastric artery. The deep circumflex artery courses behind the inguinal ligament and along the iliac crest before piercing the transverse abdominis muscle and interdigitating between that muscle and the internal oblique. Prior to its anastomosis with the musculophrenic artery, it can be relatively large, and care must be taken when these muscles are incised laterally to avoid vessel injury.
The femoral artery has three branches at the level of the inguinal ligament that serve the superficial layers of the lower abdominal wall. The superficial epigastric artery courses over the external oblique muscle lateral to the rectus muscle. The superficial circumflex artery has a lateral course inferior to the inguinal ligament, up to the iliac crest. The superficial external pudendal artery courses medially just superior to the inguinal ligament.
Because the linea alba is relatively bloodless, wound healing is thought to be somewhat impaired when midline incisions are used. In contrast, the epigastric vessels are subject to injury when a muscle splitting incision is used. Also, the deep circumflex or musculophrenic vessels can be injured when a transverse incision is carried too far laterally in the muscle bundles.
NERVES
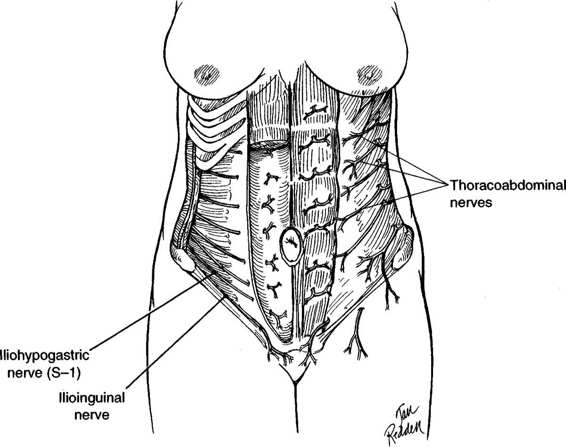
The anterior abdominal wall is supplied by the thoracoabdominal, the iliohypogastric, and the ilioinguinal nerves (Fig. 4-3). The thoracoabdominal nerves, consisting of the seventh to eleventh intercostal nerves, leave the intercostal spaces and travel caudad and anterior between the transverse abdominis and internal oblique muscles. They supply these muscles and the external oblique muscles. They also enter the sheath of the rectus and supply the rectus muscles and the overlying skin. Several trunks supply the majority of these nerves. When an incision is made lateral to the midline, a vertical type is more likely than a transverse incision to cause injury to these nerves. A vertical incision made lateral to the rectus muscle or through the muscle will denervate the tissues lying medial to the incision. The longer the incision, the greater the degree of denervation and the greater the risk of atony or atrophy of the underlying muscle.
The iliohypogastric and ilioinguinal nerves (Fig. 4-3) are sensory nerves. They course in a transverse and inferior direction just superior to the inguinal ligament. Injury to the iliohypogastric nerve, when wide transverse incisions are used, may result in sensation changes in the skin over the mons pubis, whereas injury to the ilioinguinal nerve may result in sensation changes to the labia majora. Both nerves also supply the lower fibers of the internal oblique and transverse abdominis muscles. Damage to these nerves at the level of the anterior superior iliac spine denervates these muscle fibers.
FIGURE 4-3. The major innervation of the anterior abdominal wall is via the thoracoabdominal, iliohypogastric, and ilioinguinal nerves.
WOUND HEALING
Regardless of the mechanism of injury, wound healing always includes four physiologic processes: inflammation, migration, proliferation, and maturation. The initial process, inflammation, results in hemostasis through early vasoconstriction, platelet aggregation, and clot formation. The accumulation of cellular elements results in the release of histamine from mast cells, which increases vascular permeability and causes a subsequent vasodilation. The leakage of plasma and cellular elements is clinically noted as edema. These events permit margination, migration, and diapedesis of leukocytes. Due to chemotaxis, polymorphonuclear leukocytes that phagocytize bacteria and necrotic tissue predominate for the first 3 days. Subsequently, mononuclear leukocytes appear and transform into macrophages. They not only continue phagocytosis, but also attract fibroblasts that are essential to the later proliferation process of the wound (Lebovich and Ross, 1975).
The second process of wound healing is migration. Basal cells at the wound margin proliferate and migrate across the fibrin bridge provided by the clot. The migration is controlled by contact inhibition and proceeds from the margins toward the center. This process results in the formation of a superficial layer of cells within 48 hours that is a barrier to bacterial invasion. The epithelial layer is eventually rejuvenated through proliferation and differentiation, but is relatively weak without the underlying fibroplasia that occurs simultaneously (Odland and Ross, 1968).
In the proliferation process, local mesenchymal cells differentiate into fibroblasts and migrate into the wound using the fibrin clot as scaffolding. The fibroblasts proliferate and produce mucopolysaccharides and glycoproteins that form the ground substance for fibroplasia. Within 4 days of wound creation, these cells begin producing collagen and continue to do so for up to 6 weeks. Collagen formation is responsible for the tensile strength of the wound and is ultimately the most important component of wound integrity (Howes and associates, 1929).
The proliferation process results in a disorganized array of collagen fibers. During the maturation process some of these fibers are degraded and replaced by new more organized fibers. The organized collagen fibrils undergo covalent cross-linking. This tissue remodeling and associated wound contracture are the final determinants of wound strength and appearance. The remodeling process may continue for years, but never provides the original tensile strength of the native tissue.
Disruption can occur in any of these wound-healing phases, and depends on preexisting conditions. Importantly, the fibroblastic-proliferation phase from about day 5 through day 20 provides the most strength to the wound. By day 21, most wounds will have regained almost 30 percent of their original tensile strength.
WOUND CLASSIFICATION
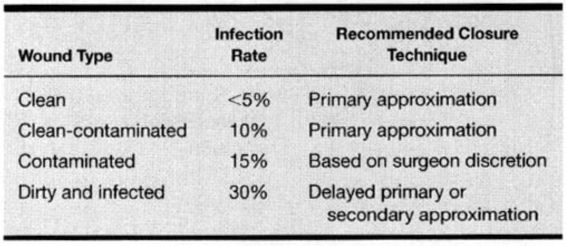
It is useful to classify operative and traumatic wounds according to the degree of bacterial contamination and asepsis that occurs at the time of the wound. The four classifications include clean, clean-contaminated, contaminated, and dirty and infected wounds. A clean wound is defined as a nontraumatic, uninfected surgical incision made under aseptic conditions without entering the genitourinary, alimentary, respiratory, or oropharyngeal tract. A clean-contaminated wound refers to a nontraumatic surgical incision that includes entry into the uninfected genitourinary, alimentary, respiratory, or oropharyngeal tract without a break in aseptic technique. Most obstetric and gynecologic wounds are categorized as clean-contaminated. A contaminated wound includes clean or clean-contaminated wounds with major breaks in aseptic technique, and surgical incisions with entry into the infected genitourinary tract or gross spillage from the alimentary tract, as well as fresh traumatic wounds. While a cesarean section performed in a laboring woman is best classified as clean-contaminated, overt chorioamnionitis and further contamination with meconium laden amniotic fluid can increase the risk to that of a contaminated wound.
Dirty and infected wounds refer to procedures for existing infection or abscesses and heavily contaminated traumatic wounds. This classification system can be used to predict the probability of postoperative wound infection and should influence the closure technique (Table 4-1). Using this classification, Culver and colleagues (1991) reviewed 84,691 operations of which 58 percent were clean, 36 percent were clean-contaminated, 4 percent were contaminated, and 2 percent were dirty or infected. The surgical wound infection rate per 100 operations was 2.1 for clean, 3.3 for clean-contaminated, 6.4 for contaminated, and 7.1 for dirty or infected wounds. Cruse and Foord (1980) published a wound infection rate as high as 7.7 percent with clean-contaminated operations. As most operations performed in obstetrics and gynecology are clean-contaminated, the surgical wound infection rate, according to the literature, is between 3.3 percent and 7.7. percent.
TABLE 4-1. Wound Classification and Associated Wound Infection
TYPES OF WOUND CLOSURE
Although incisions resulting in an excellent cosmetic scar are desirable, they are not always feasible. Abdominal wound infections, and occasionally the resulting dehiscence after obstetric operative procedures, are a psychologic and economic problem.
Most obstetric incisions are closed by primary closure. Occasionally, clinical situations occur when it is best not to perform primary incision closure. These include contaminated and dirty infected wounds, such as those with a ruptured appendix, intra-abdominal abscess, or injury to unprepared bowel. In these cases, delayed primary closure may be preferable. Following fascial closure, a bridge consisting of rolls of gauze can be used to support loosely tied, interrupted 3-0 monofilament polypropylene skin sutures (Menendez, 1985). These sutures usually are placed about 2 cm apart using a mattress technique. Dressing sponges (4 × 8 in) are laid in the wound posterior to the sutures, and are changed periodically following wound cleansing. In 5-7 days, the dressing gauze is removed, and the previously placed sutures are simply tied to approximate the skin edges. Steri-Strips may be used for further support.
If a wound infection develops anterior to the fascia and requires drainage, the wound is opened and traditionally is allowed to heal by secondary intention, although early secondary closure may be preferable to secondary intention (Hermann, 1988; Walters, 1990; and their associates). Dodson and colleagues (1992) have clearly shown that secondary closure can be performed under local anesthesia in a treatment-room setting. The infected wound is opened and debrided under local anesthesia. Saline-soaked gauzes, with or without hydrogen peroxide, are changed three times a day. Most wounds are ready for closure after 4-5 days of wound care when they appear beefy-red and are free of necrotic tissue. After local anesthesia, No. 1 polypropylene interrupted sutures, placed 3-4 cm from the skin edge, are used to reapproximate the wound and are reinforced with Steri-Strips. Still simpler is reapproximating the wound edges with an adhesive strip, which is equally efficacious as delayed secondary closure (Harris and Dodson, 1996).
FACTORS AFFECTING WOUND HEALING
Factors negatively affecting wound healing include those that inhibit the normal processes of healing. Anything that compromises normal vascularity, such as diabetes or prior irradiation, as well as anything that hinders normal metabolism, such as malnutrition, alcoholism, or chemotherapy, can compromise normal wound healing. Factors associated with increased infection, including preoperative shaving the evening before, protracted preoperative hospitalization, prolonged duration of the operation, the use of open, Penrose-type drains brought out through the incision, ascites, malignancy, and immunosuppression, also compromise wound healing. Factors that predispose to infection in obstetric patients, including prematurely ruptured membranes and long labors, also jeopardize wound integrity. Independent factors include obesity and older age (Cruse, 1977; Cruse and Foord, 1973; Dineen, 1961). Additional factors contributing to wound disruption in the absence of infection include choice of suture material, closure technique, excessive coughing, retching or vomiting, and intestinal obstruction.
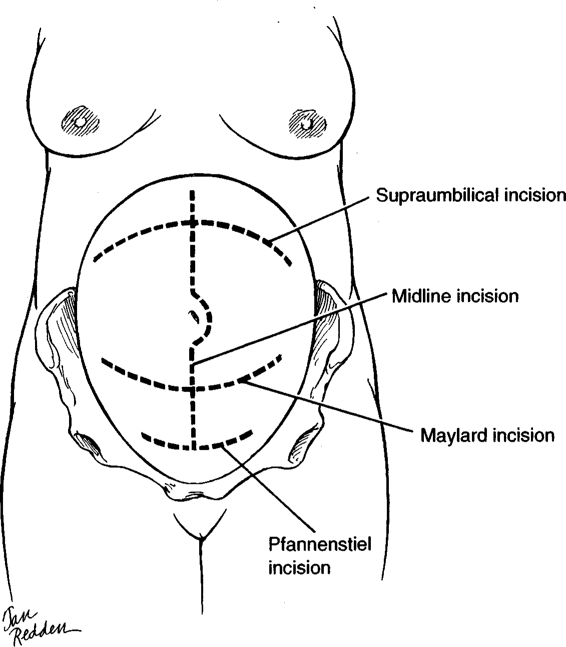
TYPES OF ABDOMINAL INCISIONS
The four incisions that are most useful for obstetric patients are shown in Figure 4-4: midline (sagittal), Pfannenstiel, Maylard, and supraumbilical (transverse). The transverse incisions achieve the best cosmetic results. Proponents of transverse incisions have suggested that they are as much as 30 times stronger than midline incisions. Mowat and Bonnar (1971), for example, observed that abdominal wound dehiscence after cesarean delivery was eight times more common with a vertical incision than with a transverse. The older literature suggests that wound evisceration was three to five times more common; and hernias developed two to three times more often when midline incisions were used (Thompson, 1949; Tollefson, 1954; and their colleagues; Helmkamp, 1977). However, some studies indicate that this increased incidence of eviscerations with midline incisions was secondary to inappropriate closures. Conversely, more recent studies either show an advantage of midline incisions over transverse incisions to avoid dehiscence, or show no difference in dehiscence rates (Farnell, 1986; Greenburg, 1979; and their colleagues).
FIGURE 4-4. The four primary abdominal incisions used in the obstetric patient, depending on the indications for operative intervention: (a) midline; (b) Pfannenstiel; (c) Maylard; and (d) supraumbilical.
TRANSVERSE INCISIONS
Transverse incisions not only produce good cosmetic results but also are less painful. In addition, when these incisions are placed in the lower abdomen, they interfere less with postoperative respiratory movement. Transverse incisions, however, do have certain disadvantages: (a) the division of multiple layers of fascia and muscle may result in the formation of dead spaces; (b) there is relatively more bleeding; (c) making the incision is relatively more time-consuming; and (d) transverse incisions may result in division of nerves (Tollefson and Russell, 1954).
PFANNENSTIEL INCISION. This is an excellent cosmetic incision and is indicated in nonobese women when the extra speed of delivery afforded by a vertical incision is not essential. The Pfannenstiel incision should not be used when exposure is critical, for example, in reexploration of a patient for hemorrhage. Exposure of the pregnant uterus often is marginal with this incision, particularly in the obese woman. Rapid extension of the incision is difficult and the extensive dissection involved often leads to diffuse small-vessel injury, which may compromise hemostasis and necessitate a subfascial closed drainage system.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree