Chapter 421 Acyanotic Congenital Heart Disease
The Obstructive Lesions
421.1 Pulmonary Valve Stenosis with Intact Ventricular Septum
Of the various forms of right ventricular outflow obstruction with an intact ventricular septum, the most common is isolated valvular pulmonary stenosis, which accounts for 7-10% of all congenital heart defects. The valve cusps are deformed to various degrees and, as a result, the valve opens incompletely during systole. The valve may be bicuspid or tricuspid and the leaflets partially fused together with an eccentric outlet. This fusion may be so severe that only a pinhole central opening remains. If the valve is not severely thickened, it produces a dome-like obstruction to right ventricular outflow during systole. Isolated infundibular or subvalvular stenosis, supravalvular pulmonary stenosis, and branch pulmonary artery stenosis are also encountered. In cases where pulmonary valve stenosis is associated with a ventricular septal defect (VSD) but without anterior deviation of the infundibular septum and overriding aorta, this condition is better classified as pulmonary stenosis with VSD rather than as tetralogy of Fallot (Chapter 424.1). Pulmonary stenosis and an atrial septal defect (ASD) are also occasionally seen as associated defects. The clinical and laboratory findings reflect the dominant lesion, but it is important to rule out any associated anomalies. Pulmonary stenosis as a result of valve dysplasia is the most common cardiac abnormality in Noonan syndrome (Chapter 76), and is associated in about 50% of cases with a mutation in the gene PTPN11, encoding the protein tyrosine phosphotase SHP-2 on chromosome 12. The mechanism for pulmonic stenosis is unknown, although maldevelopment of the distal portion of the bulbus cordis and the sequelae of fetal endocarditis have been suggested as etiologies. Pulmonary stenosis, either of the valve or the branch pulmonary arteries, is a common finding in patients with arteriohepatic dysplasia, also known as Alagille syndrome (Chapter 348). In this syndrome and in some patients with isolated pulmonic stenosis, a mutation is present in the Jagged1 gene.
Pathophysiology
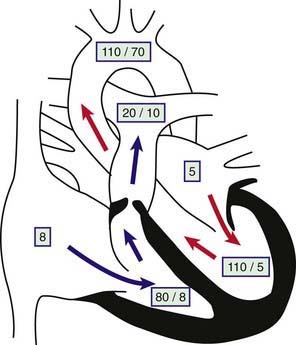
The obstruction to outflow from the right ventricle to the pulmonary artery results in increased right ventricular systolic pressure and wall stress, which leads to hypertrophy of the right ventricle (Fig. 421-1). The severity of these abnormalities depends on the size of the restricted valve opening. In severe cases, right ventricular pressure may be higher than systemic arterial systolic pressure, whereas with milder obstruction, right ventricular pressure is only mildly or moderately elevated. Pulmonary artery pressure (distal to the obstruction) is normal or decreased. Arterial oxygen saturation will be normal even in cases of severe stenosis, unless an intracardiac communication such as a VSD or ASD is allowing blood to shunt from right to left. When severe pulmonic stenosis occurs in a neonate, decreased right ventricular compliance often leads to cyanosis due to right-to-left shunting through a patent foramen ovale, a condition termed critical pulmonic stenosis.
Clinical Manifestations and Laboratory Findings
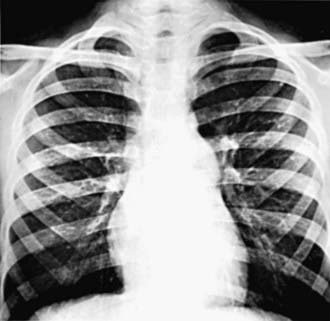
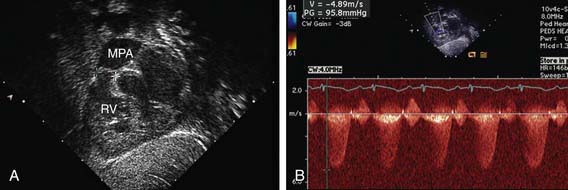
The electrocardiogram shows gross right ventricular hypertrophy, frequently accompanied by a tall, spiked P wave. Radiographic studies confirm the presence of cardiac enlargement with prominence of the right ventricle and right atrium. Prominence of the main pulmonary artery segment may be seen due to poststenotic dilatation (Fig. 421-2). Intrapulmonary vascularity is decreased. The two-dimensional echocardiogram shows severe deformity of the pulmonary valve and right ventricular hypertrophy (Fig. 421-3). In the late stages of the disease, systolic dysfunction of the right ventricle may be seen, and in these cases the ventricle may become dilated, with prominent tricuspid regurgitation. Doppler studies demonstrate a high gradient (>60 mm Hg) across the pulmonary valve. The classic findings of severe pulmonary stenosis in older children are rarely seen because of early intervention. Signs of critical pulmonic stenosis, with all of the features of severe pulmonic stenosis plus cyanosis, are usually encountered in the neonatal period.
Treatment
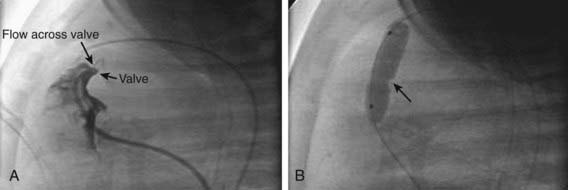
Patients with moderate or severe isolated pulmonary stenosis require relief of the obstruction. Balloon valvuloplasty is the initial treatment of choice for the majority of patients (Fig. 421-4). Patients with severely thickened pulmonic valves, especially common in those with Noonan syndrome, may require surgical intervention. In a neonate with critical pulmonic stenosis, urgent treatment by either balloon valvuloplasty or surgical valvotomy is warranted.
Crosnier C, Lykavieris P, Meunier-Rotival M, et al. Alagille syndrome. The widening spectrum of arteriohepatic dysplasia. Clin Liver Dis. 2000;4:765-778.
Feinstein JA, Kim N, Reddy VM, Perry SB. Percutaneous pulmonary valve placement in a 10-month-old patient using a hand crafted stent-mounted porcine valve. Catheter Cardiovasc Interv. 2006;67:644-649.
Khambadkone S, Coats L, Taylor A, et al. Percutaneous pulmonary valve implantation in humans: results in 59 consecutive patients. Circulation. 2005;112:1189-1197.
Krantz ID, Smith R, Colliton RP, et al. Jagged 1 mutations in patients ascertained with isolated congenital heart defects. Am J Med Genet. 1999;84:56-60.
Phoon CK. Estimation of pressure gradients by auscultation: an innovative and accurate physical examination technique. Am Heart J. 2001;141:500-506.
Rosales AM, Lock JE, Perry SB, et al. Interventional catheterization management of perioperative peripheral pulmonary stenosis: balloon angioplasty or endovascular stenting. Catheter Cardiovasc Interv. 2002;56:272-277.
Yoshida R, Hasegawa T, Hasegawa Y, et al. Protein-tyrosine phosphatase, nonreceptor type 11 mutation analysis and clinical assessment in 45 patients with Noonan syndrome. J Clin Endocrinol Metab. 2004;89:3359-3364.
421.2 Infundibular Pulmonary Stenosis and Double-Chamber Right Ventricle
Infundibular pulmonary stenosis is caused by muscular or fibrous obstruction in the outflow tract of the right ventricle. The site of obstruction may be close to the pulmonary valve or well below it; an infundibular chamber may be present between the right ventricular cavity and the pulmonary valve. In many cases, a VSD may have been present initially and later closed spontaneously. When the pulmonary valve is also stenotic, the combined defect is primarily classified as valvular stenosis with secondary infundibular hypertrophy. The hemodynamics and clinical manifestations of patients with isolated infundibular pulmonary stenosis are similar, for the most part, to those described in the discussion of isolated valvular pulmonary stenosis (Chapter 421.1).
A common variation in right ventricular outflow obstruction below the pulmonary valve is that of a double-chambered right ventricle. In this condition, a muscular band is present in the mid-right ventricular region; the band divides the chamber into two parts and creates obstruction between the inlet and outlet portions. An associated VSD that may close spontaneously is often noted. Obstruction is not usually seen early in life but may progress rapidly in a similar manner to the progressive infundibular obstruction observed with tetralogy of Fallot (Chapter 424.1).
421.3 Pulmonary Stenosis in Combination with an Intracardiac Shunt
The presence of a large left-to-right shunt at the atrial or ventricular level is evidence that the pulmonary stenosis is mild. These patients have symptoms similar to those of patients with an isolated ASD or VSD. With increasing age, worsening of the obstruction may limit the shunt and result in a gradual improvement in symptoms. Eventually, particularly in patients with pulmonary stenosis and VSD, a further increase in obstruction may lead to right-to-left shunting and cyanosis. When a patient with a VSD has evidence of decreasing heart failure and increased right ventricular forces on the electrocardiogram, one must differentiate between the development of increasing pulmonary stenosis versus the onset of pulmonary vascular disease (Eisenmenger syndrome, Chapter 427.2).
These anomalies are readily repaired surgically. Defects in the atrial or ventricular septum are closed, and the pulmonary stenosis is relieved by resection of infundibular muscle or pulmonary valvotomy, or both, as indicated. Patients with a predominant right-to-left shunt have symptoms similar to those of patients with tetralogy of Fallot (Chapter 424.1).
421.4 Peripheral Pulmonary Stenosis
A mild constriction has little effect on the pulmonary circulation. With multiple severe constrictions, pressure is increased in the right ventricle and in the pulmonary artery proximal to the site of obstruction. When the anomaly is isolated, the diagnosis is suspected by the presence of murmurs in widespread locations over the chest, either anteriorly or posteriorly. These murmurs are usually systolic ejection in quality but may be continuous. Most often, the physical signs are dominated by the associated anomaly, such as tetralogy of Fallot (Chapter 424.1).
Severe obstruction of the main pulmonary artery and its primary branches can be relieved during corrective surgery for associated lesions such as the tetralogy of Fallot or valvular pulmonary stenosis. If peripheral pulmonic stenosis is isolated, it may be treated by catheter balloon dilatation, sometimes with placement of an intravascular stent (Fig. 417-29).