Acute Head Trauma
N. Paul Rosman
Throughout the world, trauma continues to be the leading cause of death and disabilities, with most of the deaths and almost all of the severe, prolonged disabilities being caused by traumatic damage to the nervous system. Pediatric head injuries are an important contributor to this morbidity and mortality; in the United States, 2 to 5 million children sustain some degree of head trauma each year. The frequency is greater in children 5 years of age and younger than in those who are older, with head injury being the most common neurologic cause of death and disability in young children. Such injuries, occurring twice as frequently in boys as in girls, have many different causes, including falls; bicycling, skateboarding, snowboarding, and other recreational activities; competitive sports (e.g., football, ice hockey, and boxing); motor vehicle accidents; hand gun injury; and physical assault, including child abuse (with 25% of cases of head trauma in children younger than 2 years of age being due to nonaccidental trauma).
Every year in the United States, approximately 200,000 children are hospitalized with head injuries, and approximately 5,000 children die as a result of such trauma. Of these 5,000 deaths, approximately 1,500 occur from child abuse. Mortality from pediatric head injuries has been estimated to be as high as 10 per 100,000 per year. The worldwide incidence of brain injury may be as high as 500 million cases per year.
Each year in the United States, almost 30,000 persons age 19 years or younger suffer permanent disability from moderate or severe head trauma. These disabilities include posttraumatic epilepsy, cognitive impairment, learning difficulties, and behavioral and emotional problems.
In this chapter, we review the anatomic and pathophysiologic basis of head injuries in children. An approach to the diagnosis and management of acute head injuries in children is given, and the clinical disorders observed in such children are described. The prognosis in acute brain injuries and their long-term effects are discussed.
PHYSIOLOGY
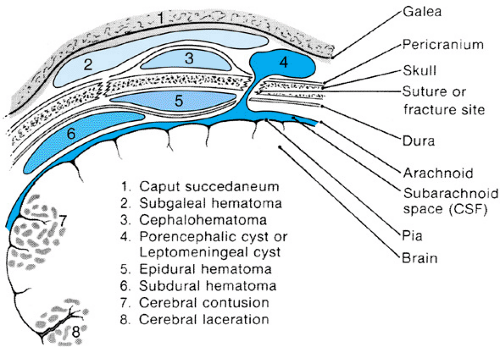
The scalp, skull, and brain all can suffer injury as a result of head trauma. Figure 118.1 depicts the brain, surrounding structures, and major associated pathologies that can complicate head trauma. The scalp, a highly vascular structure, is outermost; its inner surface is formed by the galea aponeurotica, a tendinous sheath connected anteriorly to the frontalis muscle and posteriorly to the occipitalis muscle. Beneath the galea is the subgaleal compartment, a potential space containing loose connective tissues. Next lies the skull, the outermost portion of which is the pericranium, or external periosteum. The outer and inner tables of the skull are separated by the diploic space, which is crossed by small veins. The dura lies immediately below the inner table of the skull and is relatively avascular when contrasted with the leptomeninges, which are applied closely to the brain and extend into the sulci for varying distances. Small-caliber veins within the leptomeninges cross the subdural space to drain into dural sinuses. The brain is bathed in and protected by cerebrospinal fluid (CSF), the pathways of which include the cerebral subarachnoid spaces, cisterns at the base of the brain, ventricular cavities, interconnecting channels, and foramina. The intracranial vascular system includes large vessels (e.g., internal carotid arteries and dural sinuses), intermediate-sized vessels (e.g., middle cerebral arteries and veins), and small capillaries.
Intracranial pressure (ICP) is the sum total of pressures exerted by structures within the cranium. Brain tissue, the intracranial vascular tree, and CSF play roles in determining ICP, as does the bony cranium. The skull of the newborn or older infant is not a rigid box, but rather consists of several membranous bones, with fontanelles and unfused bony structures providing outlets for the increases in ICP that commonly occur in the head-injured child. By contrast, once the cranial sutures have fused, the foramen magnum provides the only major outlet through which increases in ICP can be accommodated.
DIAGNOSIS
Patient History
Children of different ages sustain various types of head traumas. The specific circumstances of head trauma must be determined, and the predisposing factors must be identified. Such information should be sought directly from an injured child whenever possible and also from observers, such as playmates, teachers, parents, ambulance attendants, or others at the scene of the accident. Under emergency circumstances, such as posttraumatic status epilepticus or acute intracranial hemorrhage,
someone other than the person treating the injured child may be needed to obtain the history. Details of the accident should be supplemented by observations of memory loss, repetitive questioning, confusion, visual disturbance, and symptoms of increased ICP, such as altered consciousness, vomiting, severe headache, and changes in vital signs. A history of previous immunizations, drug allergies, current medications, and possible drug intoxication also should be obtained.
someone other than the person treating the injured child may be needed to obtain the history. Details of the accident should be supplemented by observations of memory loss, repetitive questioning, confusion, visual disturbance, and symptoms of increased ICP, such as altered consciousness, vomiting, severe headache, and changes in vital signs. A history of previous immunizations, drug allergies, current medications, and possible drug intoxication also should be obtained.
The principal mechanisms of head injury are contact and acceleration-deceleration. Lesions caused by an object striking the head, or vice versa, include scalp laceration, skull fracture, epidural hematoma, brain contusion, and intracerebral hemorrhage. By contrast, acceleration-deceleration, which results from head movement immediately after the injury, leads to intracranial and intracerebral pressure gradients and to shear, tensile, and compressive strains. Such inertial (nonimpact) lesions are responsible for cerebral concussion, diffuse axonal injury, and acute subdural hematoma.
General Physical Examination
Vital signs demand immediate diagnostic study and sometimes mandate emergency treatment. Characteristic changes in pulse, blood pressure, and respirations may indicate shock (i.e., decreased blood pressure and increased pulse) or increased ICP (i.e., increased blood pressure, decreased or increased pulse, slowed or irregular respirations). Usually, a decrease in blood pressure in the head-injured child is caused by an injury in another location, such as thoracic, intraabdominal, or retroperitoneal hemorrhage or bleeding into soft tissues surrounding a long-bone fracture. Occasionally, however, bleeding into the scalp may be sufficient to produce shock (as with subgaleal hematoma) or bleeding inside the skull (as with epidural hematoma). Other causes for hypotension in the head-injured child are spinal cord injury, cardiac contusion or tamponade, and tension pneumothorax.
The entire body should be scrutinized for signs of trauma. Physical examination should include a search for injury to the neck, chest, abdomen, and long bones and a careful inspection of the skin. Long-bone fractures, which occur in one-third of patients with severe head injuries, may be complicated by fat embolism. The neck should be examined with care, because of possible injury. In cases of suspected neck trauma, the neck should not be moved; the neck is immobilized by sandbags and adhesive tape or a firm collar. Such injury is suggested by cervical abrasions or cervical spine tenderness. Meningismus can result from cervical trauma, subarachnoid blood, or herniation of the cerebellar tonsils.
The head must be examined carefully. The scalp should be inspected and palpated for tenderness or depression. All scalp lacerations should be probed with a sterile-gloved finger in search of a foreign body or an underlying skull fracture. Tension of the anterior fontanelle should be assessed in young infants. Transillumination of the skull in infants and young children may detect abnormal accumulations of fluid, including blood, outside or inside the skull. Cranial ultrasound examination may be helpful when the anterior fontanelle is open. Periorbital hemorrhage (raccoon-eyes sign), ecchymosis behind the ear (Battle sign), blood behind the eardrum (hemotympanum), and bleeding from the ears or nose should be noted. These signs, along with CSF otorrhea or rhinorrhea, indicate a basilar skull fracture.
Neurologic Examination
The neurologic examination should include an assessment of the child’s alertness, orientation, and memory; a neuro-ophthalmologic examination; and testing of motor and sensory functions, reflexes, and coordination. When possible, testing of orientation and memory should include the child’s account of the episode that caused the head trauma. The presence and extent of any retrograde and anterograde (posttraumatic) amnesia should be determined. Additional assessment of memory should include testing of the child’s digit span and recall of several items after 5 minutes. A child’s repeated asking of the same question is reminiscent of Korsakoff psychosis, a posttraumatic, anterograde-type memory disturbance.
The level of consciousness may range widely. The Glasgow Coma Scale (GCS) (Table 118.1A), with scores ranging from 3 (worst) to 15 (best), provides a useful and reproducible scoring system for quantifying the level of consciousness and for systematically following a head-injured child’s clinical course. The child’s best motor response (score, 6 to 1), best verbal response (score, 5 to 1), and eye opening (score, 4 to 1) are assessed. In the young, nonverbal child, modifications in the GCS must be made. Although a variety of coma scales have been proposed for pediatric practice, a modification of the GCS that has been particularly useful is seen in Table 118.1B. In the child younger than 1 year, with eye opening, the verbal stimulus used is a shout rather than a command and for the best motor response, spontaneous movements rather than those in response to commands are assessed. With verbal response in children 5 years or younger, a score of 5 is given for appropriate words and phrases (2 to 5 years) or babbling and cooing (0 to 2 years); a 4 is given for inappropriate words (2 to 5 years) or consolable crying (0 to 2 years); a 3 is given for persistent crying or screaming to pain (0 to 5 years); a 2 is given for grunting or moaning to pain (0 to 5 years); otherwise, the same criteria as in the older child are used in scoring the younger child’s responses. Although most studies have reported a correlation between a low GCS score and severe neurologic morbidity and substantial mortality rates, children with a low GCS score (3 to 5) sometimes do surprisingly well if the head injury has not been complicated by a hypoxic-ischemic insult. Numerous factors, including intubation, orbital swelling, sedation, and neuromuscular blockade, interfere with accurate scoring with the GCS, thus limiting its predictive value.
TABLE 118.1A. GLASGOW COMA SCALE | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||
TABLE 118.1B. GLASGOW COMA SCALE MODIFIED FOR PEDIATRIC PATIENTS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Neuro-ophthalmologic evaluation should include a measurement of pupillary size and reactivity. Small pupils are seen with diencephalic and pontine injuries; a unilateral dilated pupil suggests temporal lobe herniation on the same side. The fundi should be examined carefully, with evidence of retinal and preretinal (subhyaloid) hemorrhages and papilledema specifically sought. Abnormalities of ocular gaze and position may include roving eye movements, limited lateral and vertical gaze, and skew deviation. When clearly no neck injury has occurred, the oculocephalic (doll’s-eye) maneuver can be used to assess any apparent limitation of eye movements. This procedure is performed with the child supine by rotating the head to one side and then the other. When the head is moved to the left, the eyes should deviate to the right (and vice versa) if brainstem pathways controlling eye movements are functioning normally. Lateral gaze can be tested also in the comatose patient by caloric stimulation: The child’s head is elevated 30 degrees above horizontal, and one external auditory canal is irrigated with ice water. If brainstem function is normal, the eyes should turn toward the ear being irrigated. An important precaution is ascertaining that the auditory canal is clear and that the eardrum is intact before performing this test. In an alert child, testing of visual acuity, visual fields, and opticokinetic nystagmus also should be performed.
The extent of examination of the motor system will depend on the child’s alertness. When the child is comatose, abnormal posturing (e.g., decorticate, decerebrate) should be sought. Noxious stimulation, such as a sternal rub, may be required to produce such posturing. Muscle tone may be reduced (hypotonia) or increased (spasticity or rigidity) in a hemiparetic or paraparetic distribution. In a responsive child, motor impairment can be detected by testing individual muscles, examining conventional gait and stressed gait, and observing the outstretched arms for evidence of a drift. Also, performing sensory testing may be possible. The neurologic examination is completed by testing for abnormal reflexes, such as a palmar grasp, suck, or root; by eliciting deep-tendon reflexes; and by checking for plantar responses.
Investigative Studies
Plain Radiography
The need for radiologic examination of the child with a head injury is determined by the severity of the head trauma as reflected by alterations in consciousness and by the presence or absence of focal neurologic signs. Enthusiasm for eliminating unnecessary and expensive radiologic examinations in children with minor head trauma is increasing, because of the infrequency with which abnormalities are found. Although many physicians recommend that plain skull radiography be abandoned in favor of performing immediate cranial computed tomography (CT), which is a wise approach in a severely head-injured child who needs immediate care, many fractures (e.g., linear, basilar, and facial fractures; focal depressions) are visualized better on skull radiographs than with CT. Awareness of the presence of such fractures is important; for example, a fracture of the squamous temporal bone may overlie an acute epidural hematoma, and a parietal fracture may be the site of a fracture that later begins to grow.
Severe head injury with significant loss of consciousness and focal neurologic signs (GCS score, 3 to 8) requires plain radiography and further radiologic study in most patients, after blood loss is controlled and cardiorespiratory stability is established. The initial examination in severe head trauma should include anteroposterior and lateral views of the cervical spine. It also should include anteroposterior, inclined anteroposterior (Towne), and both lateral views of the skull. At least one lateral view should be taken with a horizontal beam (cross-table) to demonstrate any air–fluid levels in the cranial cavity or in the paranasal sinuses (indicative of compound or basal fracture). If a depressed skull fracture is suspected, both standard views and tangential views of the area should be obtained. With extensive head or facial trauma, radiography should include a Waters projection of the facial bones and films of the orbits. In moderate head trauma with localizing neurologic signs or a history of loss of consciousness (GCS score 9 to 12), routine
skull radiography alone usually will suffice. When the patient has no history of neck injury, obtaining cervical spine films probably is unnecessary. In mild head trauma without focal neurologic signs or loss of consciousness (GCS score 13 to 15), usually skull and spine films are not needed. Aside from skull and spine films, additional radiography (i.e., chest, pelvis, long bones) frequently is indicated, depending on clinical circumstances.
skull radiography alone usually will suffice. When the patient has no history of neck injury, obtaining cervical spine films probably is unnecessary. In mild head trauma without focal neurologic signs or loss of consciousness (GCS score 13 to 15), usually skull and spine films are not needed. Aside from skull and spine films, additional radiography (i.e., chest, pelvis, long bones) frequently is indicated, depending on clinical circumstances.
Cranial Computed Tomography and Magnetic Resonance Imaging
In the presence of more severe head trauma or localizing neurologic signs, many imaging modalities are available, but the most helpful and least invasive is cranial CT. A cranial CT scan probably should be done also in anyone who has lost consciousness after a head injury, is amnestic for the injury, or has signs of a basilar or calvarial fracture. With persistent or progressing neurologic signs, a CT scan should be done as soon as possible, the child’s clinical state permitting. Cranial magnetic resonance imaging (MRI) has had an increasing role in the evaluation of head-injured children. Advantages of MRI include safety (no known biologic hazards and no reported side effects), the ability to image in any plane, excellent depiction of normal and pathologic anatomy, the ability to identify vessels without contrast injection (magnetic resonance angiography), and superiority to cranial CT in demonstrating the posterior fossa, where bone artifacts interfere with CT imaging. Further, MRI often will show parenchymal abnormalities in head-injured patients in whom no abnormalities are seen on CT. However, substantial limitations in using MRI with critically ill patients exist, particularly the inability to monitor such patients while they are being imaged. Thus, with acute head injuries, CT almost always is the imaging procedure of choice, whereas MRI is of greatest assistance in evaluating subacute and chronic injuries.
Usually, unilateral intracranial hemorrhage is readily evident on CT as a relatively dense mass during the immediate posttraumatic period, when the study should be performed without the infusion of a contrast medium. Recognizing acute bleeding (bleeding present within the first 1 to 3 days after injury), both extra-axial (as with subdural hemorrhage) and intra-axial (as with cerebral contusion), frequently is more difficult with MRI than with CT. This limitation arises because the deoxyhemoglobin in such lesions causes a signal that is isointense with brain on T1-weighted images and is hypointense on T2-weighted images. By contrast, edema surrounding areas of acute parenchymal hemorrhage is well-visualized with MRI because T2-weighted images of edema show high signal intensity. Because of the ease of obtaining sections in multiple planes with MRI and because no MRI signal is transmitted by bone, small collections of blood (e.g., a thin convexity extra-axial hematoma) may be visualized more easily with MRI than with CT. For this reason, MRI is more useful than is CT in imaging the posterior fossa. The sensitivity of MRI was illustrated in a series of 50 head-injured patients studied radiologically within 1 week of sustaining injury. Abnormalities indicating primary brain damage were found twice as often with MRI as with cranial CT.
After several days, blood that is broken down incompletely may be of the same density as contiguous brain; thus, if a CT scan (rather than MRI) is performed at that time, it should be done with and without intravenous iodinated contrast material. Vital signs and neurologic status should be monitored carefully because the potential for reactions to iodinated contrast is the same regardless of whether it is used for intravenous pyelography, angiography, or CT. Provision should be made for the management of these reactions at the time of injection and for several hours thereafter. When contrast is not used, and intracranial hemorrhage is not apparent, a hemorrhage still may be suspected if a deformation of ventricular structures or a shift of the midline is present. With bilateral lesions, such as acute subdural hematomas of infancy, a shift of midline structures may not occur. Bilateral intracranial hemorrhages also may produce ventricular enlargement through the blockage of the cerebral subarachnoid spaces or may cause reduction in ventricular size through compression of brain tissue. CT also can show brain edema with reduced ventricular size, loss of brain tissue with ventricular enlargement, and most skull fractures.
MRI is especially useful for detecting lesions that are isodense on CT, such as subacute extra-axial hematomas (3 to 14 days) and chronic extra-axial hematomas (greater than 14 days). Such lesions disclose high signal intensity on both T1 and T2-weighted images because of the formation of methemoglobin in subacute hematomas and increased protein content in hematomas that have become chronic. Diffuse white-matter shearing injuries are visualized very well with MRI because these lesions disclose increased signal intensity on T2-weighted images.
A great value of both CT and MRI is their use in documenting an intracranial process over the course of time. Although initial studies performed within a few hours of injury may be negative or may disclose only minor changes, follow-up studies may document changes in the size of a lesion, appearance of new lesions, brain swelling, deformation of the ventricular system, shift of midline structures, or brain herniation. Later studies also can show chronic sequelae of head injury, such as hydrocephalus and brain atrophy.
Ultrasonography
In newborns or young infants with open fontanelles and sutures, real-time cranial ultrasonography may be very useful in demonstrating intracranial blood (i.e., intraventricular, parenchymal, subarachnoid), displacement of the ventricular system, hydrocephalus, encephalomalacia, and brain calcifications.
Magnetic Resonance Angiography
In some cases of head trauma, magnetic resonance angiography may be needed to demonstrate injuries to major vessels of the head and neck. Additionally, it is helpful in providing supportive evidence for the determination of brain death.
Lumbar Puncture
Lumbar puncture (LP) should not be performed in a head-injured child unless complicating central nervous system (CNS) infection is suspected. Usually, an LP is contraindicated when significantly increased ICP is present, and one is absolutely contraindicated when evidence of an intracranial mass exists. LP may yield evidence of infection, as with meningitis complicating a basilar skull fracture; of recent subarachnoid bleeding, as with cerebral contusion; of older subarachnoid bleeding, as with xanthochromic CSF accompanying a chronic subdural hematoma; and of increased ICP, which can complicate several types of head injuries.
Subdural Taps
Subdural taps may be indicated as a diagnostic measure, as a therapeutic measure, or both. A maximum of 15 mL of fluid is removed from each subdural space without aspiration; within 1 to 2 days, the taps can be repeated.
Other Studies
In moderately and severely injured children and in those in whom the cause or circumstances of the injury are unknown, additional studies may be indicated. They include complete blood cell count; serum amylase; urinalysis; platelet and clotting studies; toxic screen on blood, urine, and gastric aspirate; and skeletal survey for old and recent fractures.
TREATMENT
General Support
The management of the child with head injury must be directed to the entire patient. The head and neck must be stabilized; with suspected neck trauma, a firm cervical collar (Philadelphia type) or sandbags or tape and Velcro straps attached to a backboard should be used. Life-threatening obstruction of the airway may result from blood, vomiting, secretions, a foreign object, or the tongue (the last especially with severe mandibular fractures). Accessible foreign objects should be removed, and patency of the airway maximized by proper positioning. Usually, a chin lift will relieve upper airway obstruction. If any concern about an accompanying cervical spine injury exists, a jaw thrust, not a chin lift, with the head kept in a neutral position, should be used to open the airway.
Ventilatory management is assisted by inserting an oral airway into the oropharynx; if needed, ventilatory support should be provided. Suctioning should be performed without touching the carina. In comatose children and in others with airway obstruction or ineffective respiratory effort with a diminished gag and cough, and in patients with a GCS of less than 8, orotracheal or nasotracheal intubation usually is needed. Nasotracheal intubation should not be used if a basal skull fracture is suspected. Intubation diminishes the risk of aspiration (of oropharyngeal secretions, blood, or vomitus) and the risk of airway obstruction, assists in tracheal suctioning, and is mandatory for controlled ventilation. The head and neck should be moved with great care, any associated neck injury is not exacerbated. Optimal conditions for intubation are provided by rapid induction of intravenous anesthesia with propofol 1 to 2 mg/kg or etomidate 1.5 to 3 mg/kg; with any hemodynamic instability, however, a combination of fentanyl, 1 to 5 μg/kg, and midazolam, 0.1 mg/kg, could be given intravenously instead. Intravenous lidocaine, 1 to 1.5 mg/kg, should be given 1 to 2 minutes before intubation, with neuromuscular blockade accomplished with intravenous high-dose vecuronium, 0.2 to 0.3 mg/kg, or vecuronium, 0.01 mg, followed by succinyl choline, 1.5 mg/kg. Oxygen (100%) should be given at 3 to 10 L/minute by bag and mask or by nasal prongs, with the PaO2 maintained at 90 to 100 mm Hg. Cardiac rate and rhythm should be monitored.
An intravenous line should be established; blood testing should include hematocrit, type and cross-match, and amylase (for evidence of pancreatic injury). Sites of hemorrhage must be controlled, and blood volume must be maintained. With infrequent exceptions, shock is not a sign of head injury in children. It usually is caused by associated injury, such as rupture of an abdominal organ (e.g., liver); bleeding into extracranial soft tissues (as with a pelvic fracture); peritonitis (after spillage of contents from a ruptured intestine); traumatic pancreatitis; or associated spinal cord injury (“spinal shock”). Occasionally, however, rapid intracranial bleeding into the epidural space or even into the scalp can cause shock. In all children with severe head injury, a central venous line to monitor central venous pressure should be placed, and an arterial line should be inserted to monitor arterial blood pressure, assess cerebral perfusion pressure, and facilitate blood gas measurements. Normal systolic blood pressure varies with age: 50 to 60 mm Hg in neonates; 70 to 80 in toddlers; 80 to 90 in school-aged children; and 90 to 100 in adolescents. Mean systemic arterial blood pressure should be maintained at 65 mm Hg at least to ensure adequate cerebral circulation. Hypovolemia (greater than 20% blood volume loss) is corrected by intravenous crystalloid (such as lactated Ringer solution or normal saline) or colloid (hydroxyethyl starch or 5% albumin in a bolus of 10 to 20 mL/kg, repeated as necessary to establish adequate perfusion).
A slowing in pulse rate may indicate an encouraging response to treatment for circulatory insufficiency, but it also can be a danger signal, reflecting increased ICP. Circulatory failure with a fall in blood pressure is treated with volume replacement, after which epinephrine, 0.1 to 0.5 μg/kg/minute, or norepinephrine, 0.05 to 0.2 μg/kg/minute, can be given. With cardiac arrest, in addition to ventilation and chest compressions, the child should be treated in accordance with the Pediatric Advanced Life Support (PALS) recommendations.
Accompanying injuries, such as those to the scalp, chest (especially pneumothorax), great vessels, abdominal viscera, pelvis, limbs, or spine, may require specific treatment. Chest films aimed at looking for rib fractures, hemopneumothorax, and mediastinal widening should be obtained. With suspected abdominal bleeding or intestinal perforation, abdominal ultrasonography, CT scanning, or diagnostic peritoneal lavage should be performed.
Pseudosubluxations of the cervical spine, especially at C2 to C3 and C3 to C4, found normally in approximately 20% of children in the first decade of life, may be sources of unnecessary concern. Fractured limbs should be splinted. The stomach should be emptied by nasogastric intubation (to prevent vomiting, aspiration, and pressure on the diaphragm, causing secondary respiratory compromise), and the bladder should be catheterized. Fever should be controlled by sponging and antipyretics. When circulatory status is adequate, the intake of iso-osmolar fluids should be given at or slightly above normal maintenance. Electrolyte abnormalities and coagulation defects should be corrected. Vital signs should be followed with care. An elevation of systolic blood pressure, a slowing or speeding of the pulse, and slowed or irregular respirations are indicative of intracranial hypertension (see section, Raised Intracranial Pressure). When seizures occur, anticonvulsants should be given (see section, Posttraumatic Seizures).
Emergency Room Management of a Child with Mild Head Trauma
Children, adolescents, and young adults frequently are evaluated in emergency departments after sustaining minor head trauma. Useful data concerning the management of such children are few in number and not uncommonly conflicting. Thus, the American Academy of Pediatrics (AAP) and the American Academy of Family Practice (AAFP) have developed a Clinical Practice Guideline to assist in managing patients between 2 and 20 years of age who have sustained an isolated, minor, closed head injury. By definition, such patients show a normal mental status on initial examination, have no focal or otherwise abnormal findings on neurologic examination, and have no physical evidence of skull fracture. Additionally, they may or may not have sustained a brief (less than a minute) loss of consciousness, they may have had a seizure immediately after their head injury, they may have vomited, and they may have developed headache and lethargy.
When no loss of consciousness has occurred in such children, following a thorough history and physical and neurologic examinations, the child should be observed for at least 24 hours in the clinic, office, emergency department, and/or at home under the care of a competent adult; careful observation should continue for the next several days, watching for any change in the patient’s clinical status. CT scanning, skull radiographs, or MRI rarely are needed. When a brief loss of consciousness has occurred following such head injury, a slightly increased risk of an intracranial injury (1% to 5% versus less than 1% with no loss of consciousness) exists. Again, following a thorough history and physical and neurologic examinations, the child should be observed in any of the above settings and/or at home under the care of a competent adult. Because of its sensitivity and specificity, cranial CT scanning may be useful in identifying the small number of such patients in whom some intracranial abnormality is present, although only rarely are medical, neurosurgical, or other interventions needed. Factors that may indicate a clearer need for CT scanning include a delay from the time of injury until the time when the patient is first seen, a scalp hematoma overlying the course of the middle meningeal artery, and those few patients with minor head trauma in whom clinical worsening occurs. Skull radiographs or MRI are almost never needed. If imaging seems desirable, cranial CT scanning is the best modality to use; if the CT scan is normal, an adverse outcome is very unlikely.
In children younger than 2 years of age who have had a minor head injury, the risk for skull fracture and intracranial injury is higher than in older patients. Additionally, clinical assessment is more difficult in very young children, and their risk for nonaccidental injury is higher. Thus, based on the assessed risk for intracranial injury, four guidelines have been suggested for the evaluation and management of children younger than 2 years old following a minor head injury:
In those determined to be of high risk for intracranial injury, a cranial CT scan should be done.
In those who show symptoms indicating possible intracranial injury, a CT scan should be done or the child should be observed for at least 4 to 6 hours post-injury, watching for any new symptoms or signs. If any develop, a CT scan should be done. If none develop, the child can be discharged home under the watchful eye of a competent adult.
When some risk for skull fracture or intracranial injury is apparent, a CT scan and/or skull radiographs should be done or, alternatively, the child should be observed for 4 to 6 hours post-injury and then discharged home if no change has occurred.
Finally, when the risk of intracranial injury appears to be very low, skull radiographs are not needed, but the child must be observed carefully in the manner described above.
It is important to stress that, whereas all the above guidelines are reasonable suggestions, proposed by health professionals knowledgeable about pediatric head injuries, these guidelines are not supported by large numbers of controlled clinical studies. In some cases, clinical judgment should take precedence, and if the clinician is concerned, even if the child looks well, CT scanning should be performed.
Hospitalization
Many factors influence the decision concerning whether to hospitalize head-injured children. Hospitalization is indicated or should be considered seriously with changing vital signs; posttraumatic seizures; altered mental status, particularly prolonged unconsciousness; persisting memory deficit; focal neurologic signs; depressed skull fracture; basilar skull fracture; enlarging scalp swelling; persisting severe headache, especially with neck stiffness; recurrent vomiting; unexplained fever; neuroradiologic abnormalities of concern; and an unexplained injury raising the question of possible child abuse.
Posttraumatic Seizures
Early posttraumatic seizures or early posttraumatic epilepsy, which occurs within the first week after head injury, develops in approximately 5% of children hospitalized after encurring a head trauma. Of these children, 20% to 30% will have additional seizures beyond the first week. Infants and young children are at greater risk for the development of early posttraumatic seizures than are older children and adults. The incidence of early posttraumatic seizures that occur after severe traumatic brain injury in children varies from 20% to 40%. The risk is greater with lower GCS scores. After adjustment for the GCS and the duration of coma, the risk of early posttraumatic seizures occurring after severe head injury is three times greater in infants than in older children to age 12 years.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree