Andrew J. Satin, MD, FACOG
INTRODUCTION, DEFINITIONS, AND EPIDEMIOLOGY
ETIOLOGY, CLINICAL PRESENTATION, AND DIAGNOSIS OF ACUTE KIDNEY INJURY
2. Acute Interstitial Nephritis
• Thrombotic Thrombocytopenia Purpura/Hemolytic Uremic Syndrome
• Acute Fatty Liver of Pregnancy
CHRONIC KIDNEY DISEASE AND PREGNANCY CONSIDERATIONS
• Physiology and Pathophysiology
• Pregnancy and Chronic Kidney Disease
INTRODUCTION, DEFINITIONS, AND EPIDEMIOLOGY
Although pregnancy-related renal failure has become a rare occurrence in developed countries, it still remains a challenge in the clinical setting and is associated with mortality and significant long-term morbidity. Original definitions of renal failure ranging from serum creatinine (sCr) levels of >0.8 mg/dL to dialysis have rendered comparison of epidemiologic studies difficult.1–5
With the introduction of the Risk, Injury, Failure, Loss, and End-stage Kidney (RIFLE) Consensus Classification for Acute Kidney Injury (RIFLE-AKI), there is now a standard description from mild to severe forms of AKI. The adverse effects of small changes in sCr have been recognized and systematically studied.2,6,7 Hence, the term AKI replaces the old term, “acute renal failure (ARF),” to encompass the entire spectrum of the syndrome, from minor changes in renal function to the requirement for dialysis.7 The RIFLE classification defines three grades of AKI severity (R—Risk, I—Injury, F—Failure) based on at least a 50% change in sCr relative to the reference sCr3,8,10 (Figure 15-1). The most recent international consensus conference expanded the RIFLE criteria to include changes as small as 0.3 mg/dL, further recognizing the negative impact of even smaller changes in sCr on short- and long-term outcomes in different patient populations.10
FIGURE 15-1. RIFLE criteria for acute kidney injury.
The incidence of pregnancy-related kidney injury has decreased from 1/3000 to 1/15,000.9 Although there has been a decline in reported pregnancy-related kidney injury, there has been little change in overall mortality and morbidity. This is likely secondary to the fact that patients that develop kidney injury are likely to have multiorgan dysfunction along with a more severe pathogenesis of disease. Associated mortality with AKI with subsequent kidney failure ranges from 0% to 30%; however, there is usually a full renal recovery in survivors of 60% to 90%.10–13 Historically, there was a bimodal incidence of renal failure in pregnancy. There was a peak in the first trimester related to the high rates of septic abortions, and then a second peak in the third trimester of pregnancy, likely related to hypertensive diseases of pregnancy. Therefore, the major decrease in pregnancy-related kidney injury is related to the legalization of abortion and an increase in surveillance for preeclampsia and other obstetric complications in the third trimester of pregnancy.10,11 Although it is anticipated that many reproductive aged women will have normal renal function, it is well known that in women with underlying chronic renal dysfunction (baseline sCr >1.4), there is a significant increased risk of renal loss (43%), and 10% of patients will experience a rapid deterioration of renal function.
ETIOLOGY, CLINICAL PRESENTATION, AND DIAGNOSIS OF ACUTE KIDNEY INJURY
Approaching the pregnant patient with kidney injury is similar to that of the nonpregnant patient. Although disease processes causing kidney injury specific to pregnancy should always be considered (ie, preeclampsia, postpartum hemorrhage, HELLP syndrome), it is still important for the high-risk obstetrician to classify his or her patients kidney disease.
AKI is traditionally classified into three subgroups: prerenal, intrinsic or intrarenal, and postrenal. Not only will these three classes of renal failure be discussed, but disease processes specific to pregnancy will also be discussed as well.
Prerenal Azotemia
Prerenal azotemia is the result of decreased renal perfusion secondary to volume depletion or low cardiac output.5 This renal hypoperfusion causes ischemia resulting in injury. In pregnancy, the most common cause of volume depletion-related kidney injury is obstetric hemorrhage, which can result from spontaneous abortion, placental abruption, placenta previa, uterine atony, and retained products of conception.5 In rare cases, severe dehydration from hyperemesis can cause volume depletion resulting in kidney injury even requiring renal replacement therapy (RRT).14,15
Severe sepsis and septic shock are also common causes of prerenal azotemia. Sepsis from any cause can lead to decreased renal perfusion, resulting in prerenal azotemia and further tubular necrosis. In pregnancy, sepsis may arise from pyelonephritis, chorioamnionitis, or endometritis. In nonobstetric patients, sepsis has consistently been found to be a leading contributing factor to kidney injury in the critically ill.16,17 Not only can sepsis precipitate kidney injury, sepsis-associated kidney injury is, in general, associated a more severe forms of AKI and an increased hospital mortality.18 Congestive heart failure is also a known cause of kidney injury. Although the exact cause of worsening renal function in heart failure is unclear, hypoperfusive injury is postulated as a mechanism for AKI as well.19
In general, prerenal azotemia can be corrected with aggressive volume resuscitation and treatment of the underlying cause. However, kidney function in the setting of heart failure should be carefully evaluated as inotropic support and afterload reduction may be needed to enhance and guide renal perfusion.
Intrinsic Renal Failure
Acute intrinsic kidney injury can result from a variety of renal diseases seen both in pregnant and nonpregnant patients. Intrinsic kidney injury may be the result of unresolved prerenal azotemia or could be the result of a direct glomerular process resulting in kidney damage. It is important for the obstetric practitioner to recognize subtle differences in these disease pathologies to steer treatment. These changes can be recognized through history, physical exam, and laboratory data involving urine sediment, and electrolytes (ie, fractional excretion of sodium [FeNa]) and ultimately may require renal biopsy (Table 15-1).
TABLE 15-1 | Categorization of AKI |
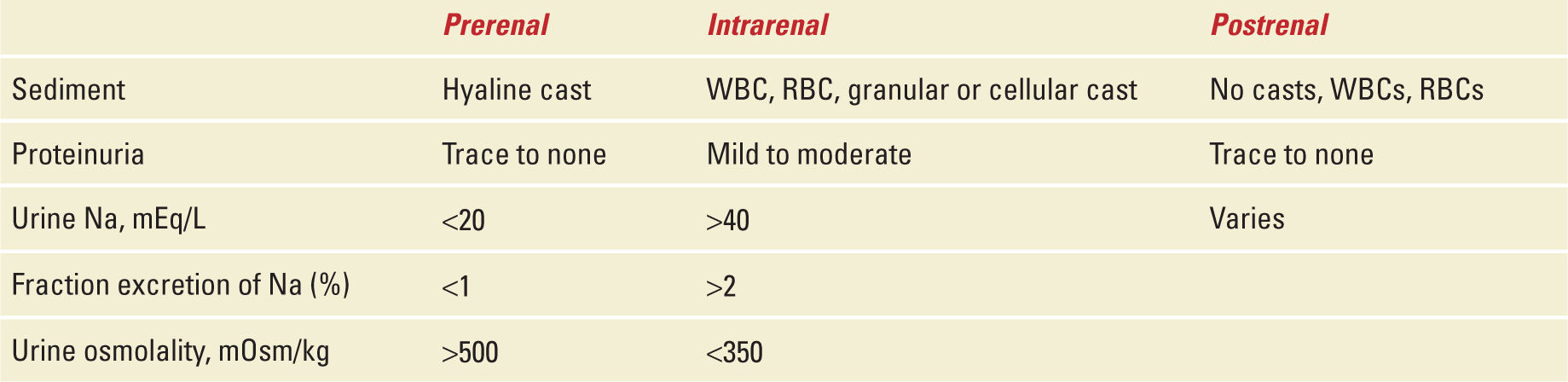
Acute Glomerulonephritis
There are numerous causes of acute glomerulonephritis including but not limited to immunoglobulin A nephropathy, poststreptococcal focal segmental glomerulonephritis, membranoproliferative glomerulonephritis, systemic lupus erythematous, polyarteritis nodosa, Wegener granulomatosis, and Goodpasture syndrome (Table 15-2). Serology including serum complement levels, antinuclear antibodies, antistreptolysin-O titers, antineutrophil cytoplasmic antibodies, and other autoantibodies may be helpful in establishing the diagnosis. Although clinical presentation can vary, patients with acute glomerulonephritis generally present with hypertension, signs of volume overload, proteinuria, and red blood cell cast in urine sediment caused by intraglomerular inflammation and cellular proliferation.20 The mainstay of treatment for acute glomerulonephritis is supportive with blood pressure control and sometimes dialysis for fluid overload. Glomerulonephritis secondary to immune-mediated disease (ie, lupus, antiglomerular basement disease) can be treated with high- and pulse-dose steroids and immunosuppresants.20 Although supportive treatment is often vital, the underlying disease process should be treated as well and often the input of a nephrologist is warranted in these situations.
TABLE 15-2 | Causes of Acute Glomeronephritis |
Acute Interstitial Nephritis
Acute interstitial nephritis (AIN) is characterized by the presence of inflammatory infiltrates and edema in the interstitium of the glomerulus that is associated with an acute deterioration of renal function. Causes of AIN are drug-induced, infection-related, and idiopathic forms (Table 15-3). AIN has also been associated with autoimmune diseases such as Sjogren syndrome, sarcoidosis, and system lupus erythematous.21 In patients with drug-induced AIN, the mean delay between start of the offending drug and appearance of renal manifestation is typically 10 days; however, the latent period can be as short as 1 day with certain antibiotics and as long as several months with nonsteroidal analgesics.22 The mainstay of treatment is removal of the offending medication or resolution of the underlying disease process. However, some studies advocate early use of corticosteroids along with plasmapheresis in severe, refractory cases.22
TABLE 15-3 | Causes of Acute Interstitial Nephritis |
Acute Tubular Necrosis
Acute tubular necrosis (ATN) is further defined by a sudden decline in glomerular filtration rate (GFR) that further inhibits the secretion of creatinine with a subsequent rise in sCr.23 ATN is caused by a variety of insults including hypoxemia, sepsis, and renal toxins (medications and contrast dye).24 However, more commonly, ATN is a disease state that is ischemic in nature caused by hypoperfusion secondary to hypovolemia (ie, dehydration, hemorrhage). It is important to delineate prerenal kidney injury from kidney injury related to ATN. This is typically done by using the FeNa. In pregnancy, ATN most commonly arises from postpartum hemorrhage in which volume resuscitation is delayed or inadequate.
ATN can be further subdivided into oliguric ATN and nonoliguric ATN. Nonoliguric ATN often but not always carries an improved prognosis compare with oliguric ATN.25 The mainstay of treatment for ATN is supportive with removal of offending agents and intravenous resuscitation. ATN like all other forms ARF may eventually require nephrology consultation and possibly RRT.
Obstruction
ARF from obstruction is usually rare in pregnancy. The gravid uterus when overdistended from polyhydramnios, multiple gestation, or uterine fibroids may cause AKI. Although it has been described in the literature from a series of case reports, this scenario is extremely rare.5 Of all of the causes of obstructive renal failure in pregnancy, kidney stones are likely the most common. The incidence of a unilateral kidney stone is the same in pregnancy as it is in the nonpregnant reproductive age female population. The work-up for renal pathology of obstructive renal failure is similar as it would be in the nonpregnant population. Risk factors for likelihood of kidney stones should be assessed, and imaging should be done at the discretion of the provider. If there is an obstruction, relief of the obstruction through cystoscopy, retrograde urethral stent placement, and percutaneous nephrostomy tubes should be explored with the help of an urologist and/or nephrologist.28
Preeclampsia/HELLP Syndrome
Preeclampsia is a pregnancy-related hypertensive disorder that has multisystem involvement. It is the most common form of hypertensive disease in pregnancy and in its severe form has renal, hepatic, neurologic, and hematologic sequela.27 In this chapter, we will focus on the renal complications of severe preeclampsia. Although a hallmark sign of preeclampsia is proteinuria, the majority of preeclamptic patients do not succumb to renal failure.5
The renal pathological finding of severe preeclampsia is glomeruloendotheliosis, which is characterized by decreased glomerular size and increased cytoplasmic volume of the glomerular endothelial cells. This results in a decreased capillary lumen diameter, which sometimes is a complete obliteration of the lumen. This in turn causes a decrease in GFR and intraluminal plasma flow, which results in prerenal AKI.28 In addition to the pathologic effects of preeclampsia at the cellular level, preeclampsia also causes relative intravascular volume depletion, vasoconstriction, and activation of inflammatory and coagulation cascade; all of which can predispose to prerenal AKI. Although the changes can occur in up to 70% of patients, the vast majority of these patients will have complete reversal of dysfunction in the postpartum period.29
As with any form of AKI, treatment must first work to treat the underlying disorder. As stated earlier, preeclampsia is progressive process; therefore, as the pregnancy continues, the renal injury can be expected to become worse or stay the same; however, improvement with continuation of pregnancy is not an expected occurrence. If kidney injury is suspected in the preeclamptic patient, volume resuscitation and adequate fluid balance are imperative. Ultimately, delivery may be necessary to prevent progression of renal injury.
Thrombotic Thrombocytopenia Purpura/Hemolytic Uremic Syndrome
Thrombotic thrombocytopenia purpura/hemolytic uremic syndrome (TTP/HUS) are disorders characterized by microangiopathic hemolytic anemia, thrombocytopenia, systemic ischemia, and multiple organ failure.30 Classically, this disease is identified by a pentad of clinical findings: thrombocytopenia, hemolytic anemia, fever, neurologic abnormalities, and renal dysfunction.30
These disorders occur in pregnancy at least as much as in the general population.31 The overall incidence of TTP/HUS in pregnancy is rare and is estimated to be at 1/25,000 births.31 Pathologically, TTP/HUS is thought to be the result of intravascular thrombi from uncleaved von Willebrand factor that can cause the consumption of platelets, fragments of red cells, and variable systemic ischemia.30 On the cellular level, large forms of von Willebrand factors are found in these patients secondary to the deficiency of the plasma protease, ADAMTS13, which cleaves these factors into the smaller nonpathologic forms. In patients with TTP/HUS, ADAMTS13 can be deficient, absent, or may be inhibited by an autoantibody.32 Although TTP/HUS can occur at any time in the antenatal or postpartum period, the most common gestational age of onset is the late second trimester around 23 weeks.30 UHS has been associated with increased endothelial injury because of complement activation secondary to a deficit of the complement inhibitor factor H. Complement inhibitors such as eculizumab improve outcomes.
The mainstay of treatment for TTP/HUS is plasmapheresis or exchange. With the use of plasmapheresis, mortality has decreased from 90% to 10%.33 Adjunctive therapies that can be considered are high-dose steroids, immunosuppressants, antiplatelet agents, and for extreme cases splenectomy; however, delivery has not shown to be of benefit.31,34–36 Even though TTP/HUS is a hematologic issue, AKI is a hallmark sign of the disease. It is imperative that not only hematologic issues be addressed but the AKI as well. This may or may not require RRT. Because of high mortality, the diagnosis of TTP/UHS should be suspected early when severe thrombocytopenia and hemolytic anemia coexist with no other alternative explanation.
Acute Fatty Liver of Pregnancy
Acute fatty liver of pregnancy (AFLP) is a disorder characterized by rapid hepatic failure in late gestation. The current incidence of AFLP is rare at 1 in 7000 births.36 Patients with AFLP clinically present with nausea, vomiting, malaise, and mental status changes. Laboratory data show elevations in liver enzymes, bilirubin levels, ammonia, hypoglycemia, and abnormal coagulation panels.37
Besides the aforementioned renal abnormalities, one can expect to see AKI as well. The renal injury seen in AFLP is multifactorial. These can be attributed to mostly prerenal insufficiency, but there is also a hypothesis of infiltration of fatty acids from affected liver hepatocytes secondary to abnormal oxidization and enzyme deficiency of 3-hydroxylacyl coenzyme A dehydrogenase.36,38 Hepatorenal syndrome may also cause AKI secondary to hepatic failure. Although there can be multiorgan dysfunction form those with AFLP, renal recovery is complete in survivors.37,38 Currently, the recommendation is for supportive therapy while awaiting swift delivery, as delivery is the only curative measure.
MANAGEMENT
When assessing and treating the patient with kidney injury, the goals of therapy should be treatment of the underlying cause, preventing further damage, and supportive therapy. Treatment can be divided into both medical management and RRT. An algorithm showing an overview of the diagnosis and management of new-onset AKI in pregnancy is shown in Figure 15-2. The mainstay of medical management depends on the volume status of the patient. For example, the hypovolemic patient usually has a more straightforward approach of volume resuscitation and correction of hypovolemia, that is, hemorrhage control. AKI in the setting of hypervolemia has a more complex approach and usually may require consultation of a nephrologist. Regardless of the volume status of patients with AKI, it is important to remove and/or avoid nephrotoxic agents and appropriately dose renally cleared medications.
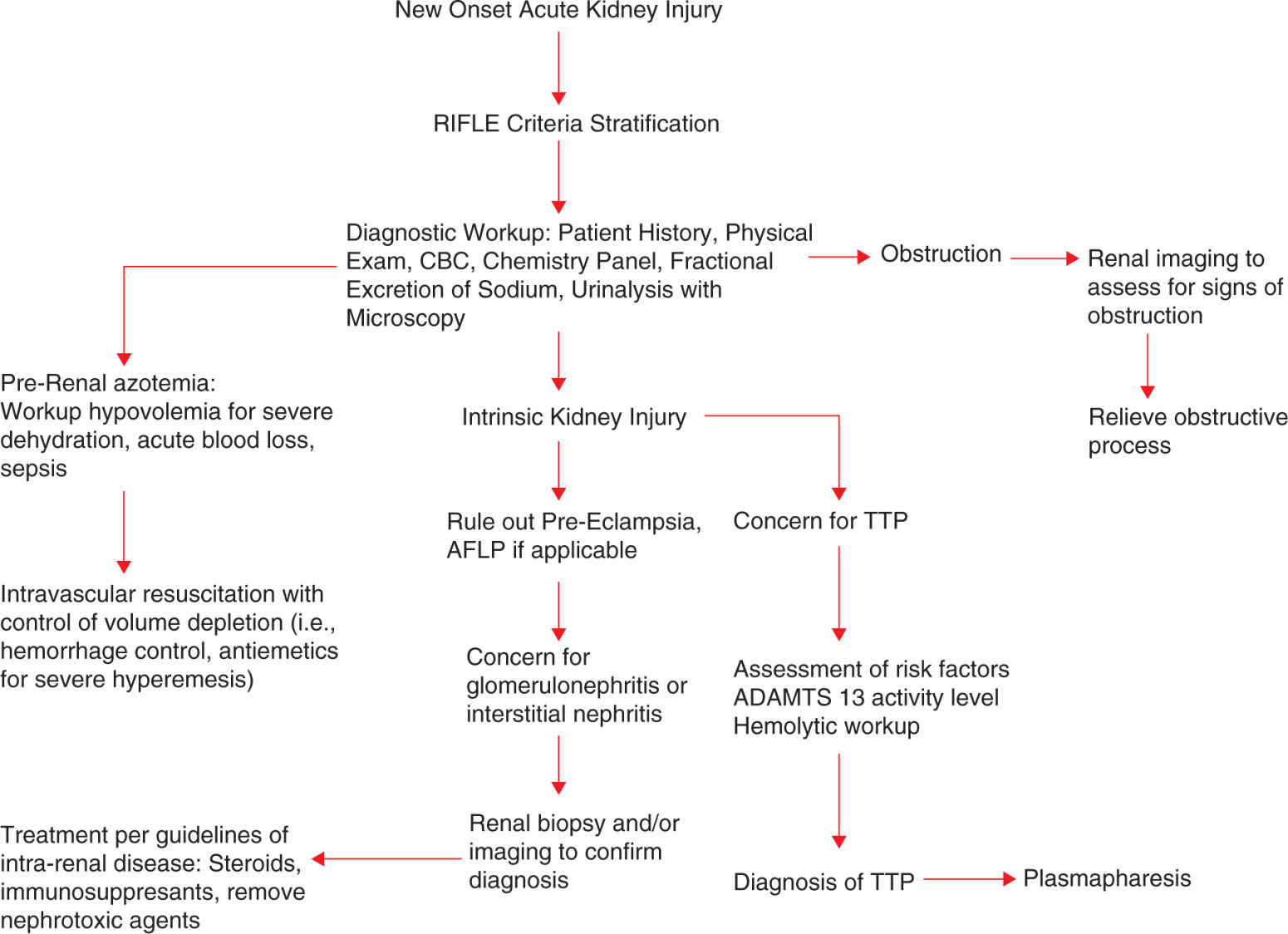
FIGURE 15-2. Initial management of the pregnant woman presenting with acute kidney disease.
In the hypervolemic patient, one of the largest mainstays of therapy is the management of volume status. There have been maneuvers to take patients from an oliguric to nonoliguric kidney injury as nonoliguric kidney injury has better outcome.39 Pharmacologic measures to transition a patient from oliguric to nonoliguric kidney injury include dopamine and the use of loop diuretics.
Low-dose dopamine, less than 5 μg/kg/min, has been thought to increase renal perfusion by causing vasodilatory effects of renal arterioles. However, current evidence suggest that there is no role for low-dose dopamine for treatment of oliguric kidney injury as it does not lower sCr levels, reduce the need for RRT, or reduce hospital stay or admission into the intensive care unit.40–43 Dopamine is a vasopressor, which can cause tachyarrhythmias, pulmonary shunting, and digital and mesenteric ischemia.39 Because of the lack of evidence that low-dose dopamine shows improved outcome in patients with kidney injury, dopamine should not be used in the management of kidney injury.
Loop diuretics have also emerged as a medical management option for the patient with AKI. Loop diuretics can increase renal intratubular flow rates, which may reduce tubular obstruction and could possibly ameliorate resulting cellular damage.43 For this reason, loop diuretics have become popular in the management of AKI. The data for loop diuretics in kidney injury are controversial. Some data suggest that diuresis does not decrease the eventual need for RRT or positively affect mortality.43 Although loop diuretics do not change outcome AKI, they are useful in the management of volume overload in the patient with AKI. Specific fetal risks of diuretic therapy have not been reported, but any agent that alters maternal hemodynamics can affect the fetus. The use of furosemide to convert the patient to a nonoliguric form of AKI does not improve outcomes and does not reduce the need for RRT. Most experts do not recommend the use of furosemide to treat oliguria.
There are a variety of reasons to start RRT (Table 15-4). Volume overload that is persistent in the setting of diuretics with end-organ effects such as pulmonary edema requires RRT. Hyperkalemia despite administration of potassium-binding resins, insulin, glucose, sodium bicarbonate, and intravenous calcium requires RRT.44 Hyperkalemic electrocardiograph (ECG) changes include peaked T waves, prolonged PR interval, and QRS duration. This can progress to bradycardia, ventricular standstill, and asystole in severe cases of hyperkalemia. Progression and severity of ECG changes do not correlate well with serum potassium concentrations. Many patients with hyperkalemia or serum potassium of greater than or equal to 6.0, but less than 7.0 still had manifestations of ECG changes.45 ECG manifestations are more likely to occur with rapid onset hyperkalemia and in the presence of hypocalcemia, acidemia, and hyponatremia. Therefore, other electrolytes abnormalities should be evaluated and corrected.45–47 Severe hyperkalemia, even without electrocardiographic changes, requires RRT.
TABLE 15-4 | Indications for RRT |




