Pathogenesis
More than 80 percent of spontaneous abortions occur within the first 12 weeks of gestation. With first-trimester losses, death of the embryo or fetus nearly always precedes spontaneous expulsion. Death is usually accompanied by hemorrhage into the decidua basalis. This is followed by adjacent tissue necrosis that stimulates uterine contractions and expulsion. An intact gestational sac is usually filled with fluid and may or may not contain an embryo or fetus. Thus, the key to determining the cause of early miscarriage is to ascertain the cause of fetal death. In contradistinction, in later pregnancy losses, the fetus usually does not die before expulsion, and thus other explanations are sought.
 Incidence
Incidence
Statistics regarding the incidence of spontaneous abortion vary according to the diligence used for its recognition. Wilcox and colleagues (1988) studied 221 healthy women through 707 menstrual cycles and found that 31 percent of pregnancies were lost after implantation. They used highly specific assays for minute concentrations of maternal serum β-hCG and reported that two thirds of these early losses were clinically silent.
Currently, there are factors known to influence clinically apparent spontaneous abortion, however, it is unknown if these same factors affect clinically silent miscarriages. By way of example, the rate of clinical miscarriages is almost doubled when either parent is older than 40 years (Gracia, 2005; Kleinhaus, 2006). But, it is not known if clinically silent miscarriages are similarly affected by parental age.
 Fetal Factors
Fetal Factors
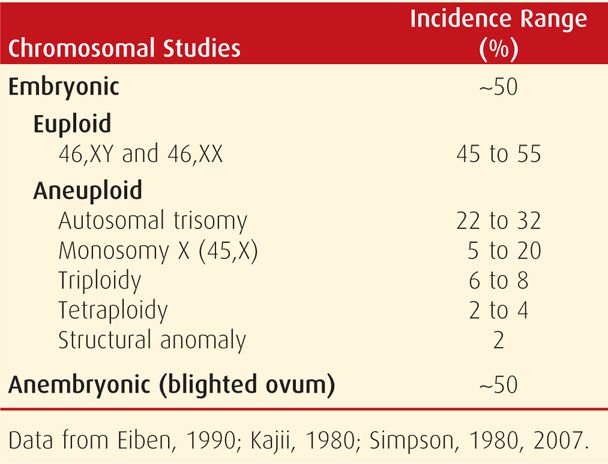
As shown in Table 18-1, approximately half of miscarriages are anembryonic, that is, with no identifiable embryonic elements. Less accurately, the term blighted ovum may be used (Silver, 2011). The other 50 percent are embryonic miscarriages, which commonly display a developmental abnormality of the zygote, embryo, fetus, or at times, the placenta. Of embryonic miscarriage, half of these—25 percent of all abortuses—have chromosomal anomalies and thus are aneuploid abortions. The remaining cases are euploid abortions, that is, carrying a normal chromosomal complement.
TABLE 18-1. Chromosomal Findings in First-Trimester Abortuses
Aneuploid Abortion
Both abortion rates and chromosomal anomalies decrease with advancing gestational age. As shown in Figure 18-1, 50 percent of embryonic abortions are aneuploid, but chromosomal abnormalities are found in just a third of second-trimester fetal losses and in only 5 percent of third-trimester stillbirths. Aneuploid abortion occurs at earlier gestational ages. Kajii and associates (1980) noted that 75 percent of aneuploid abortions occurred by 8 weeks. Of these, 95 percent of chromosomal abnormalities are caused by maternal gametogenesis errors, and 5 percent by paternal errors (Jacobs, 1980). Some found most common are listed in Table 18-1.
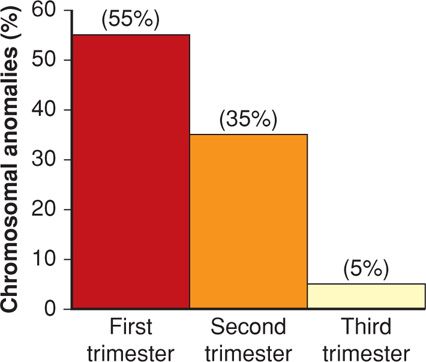
FIGURE 18-1 Frequency of chromosomal anomalies in abortuses and stillbirths during each trimester. Approximate percentages for each group are shown. (Data from Eiben, 1990; Fantel, 1980; Warburton, 1980.)
With first-trimester miscarriages, autosomal trisomy is the most frequently identified chromosomal anomaly. Although most trisomies result from isolated nondisjunction, balanced structural chromosomal rearrangements are found in one partner in 2 to 4 percent of couples with recurrent miscarriages. Trisomies have been identified in abortuses for all except chromosome number 1, and those with 13, 16, 18, 21, and 22 are most common. A previous miscarriage increases the baseline risk for aneuploidy in a subsequent fetus from 1.4 to 1.7 percent (Bianco, 2006). With two or three previous miscarriages, the risk increases to 1.8 and 2.2 percent, respectively.
Monosomy X (45,X) is the single most frequent specific chromosomal abnormality. This is Turner syndrome, which usually results in abortion, but liveborn females are described (Chap. 13, p. 264). Conversely, autosomal monosomy is rare and incompatible with life.
Triploidy is often associated with hydropic or molar placental degeneration (Chap. 20, p. 398). The fetus within a partial hydatidiform mole frequently aborts early, and the few carried longer are all grossly deformed. Advanced maternal and paternal age do not increase the incidence of triploidy. Tetraploid fetuses most often abort early in gestation, and they are rarely liveborn. Last, chromosomal structural abnormalities infrequently cause abortion.
Euploid Abortion
Chromosomally normal fetuses abort later than those that are aneuploid. Specifically, the rate of euploid abortions peaks at approximately 13 weeks (Kajii, 1980). In addition, the incidence of euploid abortions increases dramatically after maternal age exceeds 35 years (Stein, 1980).
 Maternal Factors
Maternal Factors
The causes of euploid abortions are poorly understood, but various medical disorders, environmental conditions, and developmental abnormalities have been implicated. One example is the well-known influence of maternal age just described.
Infections
Some common viral, bacterial, and other infectious agents that invade the normal human can cause pregnancy loss. Many are systemic and infect the fetoplacental unit by blood-borne organisms. Others may infect locally through genitourinary infection or colonization. However, despite the numerous infections acquired in pregnancy, these uncommonly cause early abortion. Brucella abortus, Campylobacter fetus, and Toxoplasma gondii infections cause abortion in livestock, but their role in human pregnancy is less clear (Feldman, 2010; Hide, 2009; Mohammad, 2011; Vilchez, 2014). There appear to be no abortifacient effects of infections caused by Listeria monocytogenes, parvovirus, cytomegalovirus, or herpes simplex virus (Brown, 1997; Feldman, 2010). One possible exception is infection with Chlamydia trachomatis, which was found to be present in 4 percent of abortuses compared with < 1 percent of controls (Baud, 2011). Another is polymicrobial infection from periodontal disease that has been linked with a two- to fourfold increased risk (Holbrook, 2004; Moore, 2004; Xiong, 2007).
Data concerning a link between some other infections and increased abortion are conflicting. Examples are Mycoplasma and Ureaplasma (Quinn, 1983a,b; Temmerman, 1992). Another is an association with human immunodeficiency virus (HIV) (Quinn, 1983a,b; van Benthem, 2000). Oakeshott and coworkers (2002) reported an association between second-, but not first-, trimester miscarriage and bacterial vaginosis.
Medical Disorders
In general, early abortions are rarely due to chronic wasting diseases such as tuberculosis or carcinomatosis. There are a few specific disorders possibly linked with increased early pregnancy loss. Those associated with diabetes mellitus and thyroid disease are discussed subsequently. Another example is celiac disease, which has been reported to cause recurrent abortions as well as both male and female infertility (Sharshiner, 2013; Sher, 1994). Unrepaired cyanotic heart disease is likely a risk for abortion, and in some, this may persist after repair (Canobbio, 1996). Eating disorders—anorexia nervosa and bulimia nervosa—have been linked with subfertility, preterm delivery, and fetal-growth restriction. Their association with miscarriage, however, is less well studied (Andersen, 2009; Sollid, 2004). Inflammatory bowel disease and systemic lupus erythematosus may increase the risk (Al Arfaj, 2010; Khashan, 2012). Chronic hypertension does not appear to confer significant risk (Ankumah, 2013). Perhaps related, women with a history of recurrent miscarriages were reported to be at increased risk for fetal-growth restriction (Catov, 2008). Another possible link with vascular disease is that women with multiple miscarriages are more likely to later suffer a myocardial infarction (Kharazmi, 2011).
Medications. Only a few medications have been evaluated concerning a role with early pregnancy loss. Oral contraceptives or spermicidal agents used in contraceptive creams and jellies are not associated with an increased miscarriage rate. Similarly, nonsteroidal antiinflammatory drugs or ondansetron are not linked (Edwards, 2012; Pasternak, 2013). A pregnancy with an intrauterine device (IUD) in situ has an increased risk of abortion and specifically of septic abortion (Chap. 38, p. 700). With the newer IUDs, Moschos and Twickler (2011) reported that only 6 of 26 intact pregnancies aborted before 20 weeks. Finally, studies have shown no increase in pregnancy loss rates with meningococcal conjugate or trivalent inactivated influenza vaccines (Irving, 2013; Zheteyeva, 2013).
Cancer. Therapeutic doses of radiation are undeniably abortifacient, but doses that cause abortion are not precisely known (Chap. 46, p. 930). According to Brent (2009), exposure to < 5 rads does not increase the risk.
Cancer survivors who were previously treated with abdominopelvic radiotherapy may later be at increased risk for miscarriage. Wo and Viswanathan (2009) reported an associated two- to eightfold increased risk for miscarriages, low-birthweight and growth-restricted infants, preterm delivery, and perinatal mortality in women previously treated with radiotherapy. Hudson (2010) found an associated increased risk for miscarriage in those given radiotherapy and chemotherapy in the past for a childhood cancer.
The effects of chemotherapy in causing abortion are not well defined (Chap. 12, p. 248). Particularly worrisome are women with an early normal gestation erroneously treated with methotrexate for an ectopic pregnancy (Chap. 19, p. 384). In a report of eight such cases, two viable-size fetuses had multiple malformations. In the remaining six cases, three each had a spontaneous or induced abortion (Nurmohamed, 2011).
Diabetes Mellitus
The abortifacient effects of uncontrolled diabetes are well-known. Optimal glycemic control will mitigate much of this loss and is discussed in Chapters 8 (p. 157) and 57 (p. 1128). Spontaneous abortion and major congenital malformation rates are both increased in women with insulin-dependent diabetes. This is directly related to the degree of periconceptional glycemic and metabolic control.
Thyroid Disorders
These have long been suspected to cause early pregnancy loss and other adverse pregnancy outcomes. Severe iodine deficiency, which is infrequent in developed countries, has been associated with increased miscarriage rates (Castañeda, 2002). Varying degrees of thyroid hormone insufficiency are common in women. Although the worst—overt hypothyroidism—is infrequent in pregnancy, subclinical hypothyroidism has an incidence of 2 to 3 percent (Casey, 2005; Garber, 2012). Both are usually caused by autoimmune Hashimoto thyroiditis, in which both incidence and severity accrue with age. Despite this common prevalence, any increased risks for miscarriage due to hypothyroidism are still unclear (Krassas, 2010; Negro, 2010). That said, De Vivo (2010) reported that subclinical thyroid hormone deficiency may be associated with very early pregnancy loss.
The prevalence of abnormally high serum levels of antibodies to thyroid peroxidase or thyroglobulin is nearly 15 percent in pregnant women (Abbassi-Ghanavati, 2010; Haddow, 2011). Although most of these women are euthyroid, those with clinical hypothyroidism tend to have higher concentrations of antibodies. Even in euthyroid women, however, antibodies are a marker for increased miscarriage (Benhadi, 2009; Chen, 2011; Thangaratinam, 2011). This has been confirmed by two prospective studies, and preliminary data from one suggest that thyroxine supplementation decreases this risk (Männistö, 2009; Negro, 2006). Effects associated with thyroid disorders in women with recurrent miscarriage are considered further on page 359.
Surgical Procedures
The risk of miscarriage caused by surgery is not well studied. There is extensive interest in pregnancy outcomes following bariatric surgery, because as discussed on page 353, obesity is an uncontested risk factor for miscarriage. However, currently, it is not known if this risk is mitigated by weight-reduction surgery (Guelinckx, 2009).
It is likely that uncomplicated surgical procedures performed during early pregnancy do not increase the risk for abortion (Mazze, 1989). Ovarian tumors can generally be resected without causing miscarriage (Chap. 63, p. 1227). An important exception involves early removal of the corpus luteum or the ovary in which it resides. If performed before 10 weeks’ gestation, supplemental progesterone should be given. Between 8 and 10 weeks, a single 150-mg intramuscular injection of 17-hydroxyprogesterone caproate is given at the time of surgery. If between 6 to 8 weeks, then two additional 150-mg injections should be given 1 and 2 weeks after the first. Other progesterone regimens include: (1) oral micronized progesterone (Prometrium), 200 or 300 mg orally once daily, or (2) 8-percent progesterone vaginal gel (Crinone) given intravaginally as one premeasured applicator daily plus micronized progesterone 100 or 200 mg orally once daily continued until 10 weeks’ gestation.
Trauma seldom causes first-trimester miscarriage, and although Parkland Hospital is a busy trauma center, this is an infrequent association. Major trauma—especially abdominal—can cause fetal loss, but is more likely as pregnancy advances (Chap. 47, p. 950).
Nutrition
Extremes of nutrition—severe dietary deficiency and morbid obesity—are associated with increased miscarriage risks. Dietary quality may also be important, as this risk may be reduced in women who consume fresh fruit and vegetables daily (Maconochie, 2007).
Sole deficiency of one nutrient or moderate deficiency of all does not appear to increase risks for abortion. Even in extreme cases—for example, hyperemesis gravidarum—abortion is rare (Maconochie, 2007). Other examples discussed on page 352 are anorexia and bulimia nervosa. Importantly, Bulik and colleagues (2010) reported that half of pregnancies in women with anorexia nervosa were unplanned.
Obesity is associated with a litany of adverse pregnancy outcomes (Chap. 48, p. 965). These include subfertility and an increased risk of miscarriage and recurrent abortion (Jarvie, 2010; Lashen, 2004; Satpathy, 2008). In a study of 6500 women who conceived with in vitro fertilization (IVF), live birth rates were reduced progressively for each body mass index (BMI) unit increase (Bellver, 2010a). As noted earlier, although the risks for many adverse late-pregnancy outcomes are decreased after bariatric surgery, any salutary effects on the miscarriage rate are not clear (Guelinckx, 2009).
Social and Behavioral Factors
Lifestyle choices reputed to be associated with an increased miscarriage risk are most commonly related to chronic and especially heavy use of legal substances. The most common used is alcohol, with its potent teratogenic effects discussed in Chapter 12 (p. 245). That said, an increased miscarriage risk is only seen with regular or heavy use (Floyd, 1999; Maconochie, 2007). In fact, low-level alcohol consumption does not significantly increase the abortion risk (Cavallo, 1995; Kesmodel, 2002).
At least 15 percent of pregnant women admit to cigarette smoking (Centers for Disease Control and Prevention, 2013). It seems intuitive, but unproven, that cigarettes could cause early pregnancy loss by a number of mechanisms that cause adverse late-pregnancy outcomes (Catov, 2008).
Excessive caffeine consumption—not well defined—has been associated with an increased abortion risk. There are reports that heavy intake of approximately five cups of coffee per day—about 500 mg of caffeine—slightly increases the abortion risk (Armstrong, 1992; Cnattingus, 2000; Klebanoff, 1999). Studies of “moderate”—less than 200 mg daily—did not increase the risk (Savitz, 2008; Weng, 2008). Currently, the American College of Obstetricians and Gynecologists (2013b) has concluded that moderate consumption likely is not a major abortion risk and that any associated risk with higher intake is unsettled. Adverse effects of illicit drugs are discussed in Chapter 12 (p. 253).
Occupational and Environmental Factors
It is intuitive to limit exposure of pregnant women to any toxin. That said, although some environmental toxins such as benzene are implicated in fetal malformations, data with miscarriage risk is less clear (Lupo, 2011). The major reason is that it is not possible to accurately assess environmental exposures. Earlier reports that implicated some chemicals as increasing miscarriage risk include arsenic, lead, formaldehyde, benzene, and ethylene oxide (Barlow, 1982). More recently, there is evidence that DDT—dichlorodiphenyltrichloroethane—may cause excessive miscarriage rates (Eskenazi, 2009). In fact, use of DDT-containing insecticides had been suspended. But in 2006, it was again and is still endorsed by the World Health Organization (2011) for mosquito control for malaria prevention.
There are even fewer studies of occupational exposures and abortion risks. In a follow-up of the Nurses Health Study II, Lawson and associates (2012) reported slightly increased miscarriage risks in nurses exposed to antineoplastic drugs, sterilizing agents, and x-rays. Some of these found that exposure to video display terminals or to ultrasound did not increase miscarriage rates (Schnorr, 1991; Taskinen, 1990). Increased miscarriage risk was found for dental assistants exposed to more than 3 hours of nitrous oxide daily if there was no gas-scavenging equipment (Boivin, 1997; Rowland, 1995). Conclusions from a metaanalysis were that there is a small incremental risk for spontaneous abortion in women who worked with cytotoxic antineoplastic chemotherapeutic agents (Dranitsaris, 2005).
Immunological Factors
The immune tolerance of the mother to the paternal-haploid fetal combination remains enigmatic (Calleja-Agius, 2011; Williams, 2012). This is discussed in greater detail in Chapter 5 (p. 97). There is, however, an increased risk for early pregnancy loss with some immune-mediated disorders. The most potent of these are antiphospholipid antibodies directed against binding proteins in plasma (Erkan, 2011). These along with clinical and laboratory findings provide criteria for the antiphospholipid antibody syndrome—APS (American College of Obstetricians and Gynecologists, 2012). Because associated pregnancy loss can be repetitive, recurrent miscarriage due to APS is discussed on page 359.
Inherited Thrombophilias
Although thrombophilias were initially linked to various pregnancy outcomes, most putative associations have been refuted. Currently, the American College of Obstetricians and Gynecologists (2013a) is of the opinion that there is not a definitive causal link between these thrombophilias and adverse pregnancy outcomes in general, and abortion in particular.
Uterine Defects
Various inherited and acquired uterine defects are known to cause both early and late recurrent miscarriages, and they are considered on page 358.
 Paternal Factors
Paternal Factors
These factors in the genesis of miscarriage are not well studied. Chromosomal abnormalities in sperm reportedly had an increased abortion risk (Carrell, 2003). Increasing paternal age was significantly associated with increased risk for abortion in the Jerusalem Perinatal Study (Kleinhaus, 2006). This risk was lowest before age 25 years, after which it progressively increased at 5-year intervals.
 Clinical Classification of Spontaneous Abortion
Clinical Classification of Spontaneous Abortion
Threatened Abortion
The clinical diagnosis of threatened abortion is presumed when bloody vaginal discharge or bleeding appears through a closed cervical os during the first 20 weeks (Hasan, 2009). Bleeding in early pregnancy must be differentiated from implantation bleeding, which some women have at the time of the expected menses (Chap. 5, p. 90). Almost a fourth of women develop clinically significant bleeding during early gestation that may persist for days or weeks. With miscarriage, bleeding usually begins first, and cramping abdominal pain follows hours to days later. There may be low-midline clearly rhythmic cramps; persistent low backache with pelvic pressure; or dull and midline suprapubic discomfort. Bleeding is by far the most predictive risk factor for pregnancy loss (Eddleman, 2006). Overall, approximately half will abort, but this risk is substantially less if there is fetal cardiac activity (Tongsong, 1995).
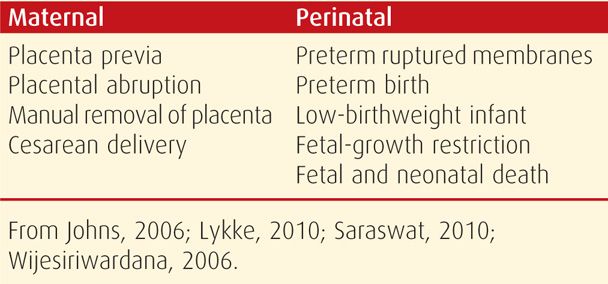
Even if miscarriage does not follow early bleeding, the risk for later adverse pregnancy outcomes is increased as shown in Table 18-2. In the study of almost 1.8 million pregnancies from the Danish National Patient Registry, there was a threefold risk for many of these pregnancy complications.
TABLE 18-2. Adverse Outcomes That are Increased in Women with Threatened Abortion
Threatened Abortion versus Ectopic Pregnancy. Every woman with an early pregnancy, vaginal bleeding, and pain should be evaluated. The primary goal is prompt diagnosis of an ectopic pregnancy. As discussed in Chapter 19 (p. 381), serial quantitative serum β-hCG and progesterone levels and transvaginal sonography are used to ascertain if there is an intrauterine live fetus. Because these are not 100-percent accurate to confirm early fetal death or location, repeat evaluations are often necessary. Serum hCG levels in women with bleeding who went on to have an early miscarriage are shown in Figure 18-2 and in Table 19-1 (p. 381). Values for women with early pregnancy bleeding who went on to have a normal pregnancy are shown in Figure 18-3. Several predictive models have been described (Barnhart, 2010; Condous, 2007; Connolly, 2013). With a robust uterine pregnancy, serum β-hCG levels should increase at least 53 to 66 percent every 48 hours (Barnhart, 2004a; Kadar, 1982). Serum progesterone concentrations < 5 ng/mL suggest a dying pregnancy, whereas values > 20 ng/mL support the diagnosis of a healthy pregnancy.
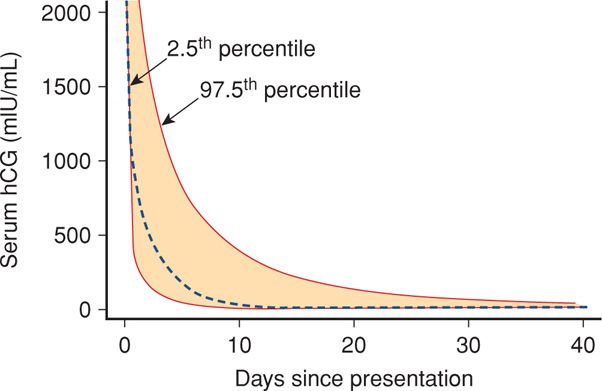
FIGURE 18-2 Composite curve describing decline in serial human chorionic gonadotropin (hCG) values starting at a level of 2000 mIU/mL following early spontaneous miscarriage. The dashed line is the predicted curve based on the summary of data from all women. The colored area within the dashed lines represents the 95-percent confidence intervals. (Data from Barnhart, 2004a.)
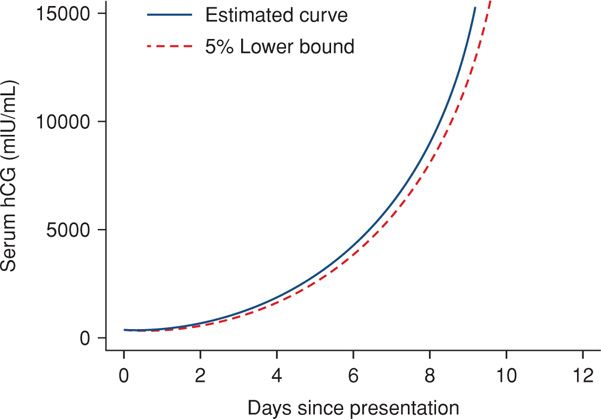
FIGURE 18-3 Composite curve of increasing serum levels of beta-human chorionic gonadotropin (β-hCG) in women with early bleeding and subsequent normal pregnancy. (Data from Barnhart, 2004b.)
Transvaginal sonography is used to locate the pregnancy and determine if the fetus is alive. If this cannot be done, then pregnancy of unknown location is diagnosed (Chap. 19, p. 381). The gestational sac—an anechoic fluid collection that represents the exocoelomic cavity—may be seen by 4.5 weeks (Fig. 9-3, p. 170). At this same time, β-hCG levels are generally considered to be 1500 to 2000 mIU/mL (Barnhart, 1994; Timor-Tritsch, 1988). Connolly and colleagues (2013) observed that this value could be as low as 390 mIU/mL, but also noted that a threshold as high as 3500 mIU/mL may be needed to identify the gestational sac in 99 percent of cases.
Another caveat is that a gestational sac may appear similar to other intrauterine fluid accumulations—the so-called pseudogestational sac (Fig. 19-5, p. 382). This pseudosac may be seen with ectopic pregnancy and is easier to exclude once a yolk sac is seen. Typically, the yolk sac is visible by 5.5 weeks and with a mean gestational-sac diameter of 10 mm. Thus, the diagnosis of a uterine pregnancy should be made cautiously if the yolk sac is not yet seen (American College of Obstetricians and Gynecologists, 2011e).
At 5 to 6 weeks, a 1- to 2-mm embryo adjacent to the yolk sac can be seen (Daya, 1993). Absence of an embryo in a sac with a mean sac diameter of 16 to 20 mm suggests a dead fetus (Levi, 1988; Nyberg, 1987). Finally, fetal cardiac activity can be detected at 6 to 6.5 weeks with an embryonic length of 1 to 5 mm and a mean sac diameter of 13 to 18 mm. A 5-mm embryo without cardiac activity is likely dead (Goldstein, 1992; Levi, 1990).
There are various management schemes derived from these findings. At Parkland Hospital, to ensure that live intrauterine pregnancies are not interrupted, we define the threshold of embryo fetal death based on values two standard deviations from the mean. Thus, an anembryonic gestation is diagnosed when the mean gestational sac diameter measures ≥ 20 mm and no embryo is seen. Embryonic death is also diagnosed if an embryo measuring ≥ 10 mm has no cardiac activity.
Management. Acetaminophen-based analgesia will help relieve discomfort from cramping. If uterine evacuation is not indicated, bed rest is often recommended but does not improve outcomes. Neither has treatment with a host of medications that include chorionic gonadotropin (Devaseelan, 2010). With persistent or heavy bleeding, the hematocrit is determined. If there is significant anemia or hypovolemia, then pregnancy evacuation is generally indicated. In these cases in which there is a live fetus, some choose transfusion and further observation.
Anti-D Immunoglobulin. With spontaneous miscarriage, 2 percent of Rh D-negative women will become alloimmunized if not provided passive isoimmunization. With an induced abortion, this rate may reach 5 percent. The American College of Obstetricians and Gynecologists (2013c) recommends anti-Rh0 (D) immunoglobulin given as 300 μg intramuscularly (IM) for all gestational ages, or 50 μg IM for pregnancies ≤ 12 weeks and 300 μg for ≥ 13 weeks.
With threatened abortion, immunoglobulin prophylaxis is controversial because of sparse evidence-based data (American College of Obstetricians and Gynecologists, 2013c; Hannafin, 2006; Weiss, 2002). That said, some choose to administer anti-D immunoglobulin up to 12 weeks’ gestation for a threatened abortion and a live fetus. At Parkland Hospital, we administer a 50-μg dose to all Rh D-negative women with first-trimester bleeding.
Inevitable Abortion
In the first trimester, gross rupture of the membranes along with cervical dilatation is nearly always followed by either uterine contractions or infection. A gush of vaginal fluid during the first half of pregnancy usually has serious consequences. In some cases not associated with pain, fever, or bleeding, fluid may have collected previously between the amnion and chorion. If this is documented, then diminished activity with observation is a reasonable course. After 48 hours, if no additional amnionic fluid has escaped and if there is no bleeding, cramping, or fever, then a woman may resume ambulation and pelvic rest. With bleeding, cramping, or fever, abortion is considered inevitable, and the uterus is evacuated.
Incomplete Abortion
Bleeding that follows partial or complete placental separation and dilation of the cervical os is termed incomplete abortion. The fetus and the placenta may remain entirely within the uterus or partially extrude through the dilated os. Before 10 weeks, they are frequently expelled together, but later, they deliver separately. Management options of incomplete abortion include curettage, medical abortion, or expectant management in clinically stable women as discussed on page 357. With surgical therapy, additional cervical dilatation may be necessary before suction curettage. In others, retained placental tissue simply lies loosely within the cervical canal and can be easily extracted with ring forceps.
Complete Abortion
At times, expulsion of the entire pregnancy may be completed before a woman presents to the hospital. A history of heavy bleeding, cramping, and passage of tissue or a fetus is common. Importantly, during examination, the cervical os is closed. Patients are encouraged to bring in passed tissue, which may be a complete gestation, blood clots, or a decidual cast. The last is a layer of endometrium in the shape of the uterine cavity that when sloughed can appear as a collapsed sac (Fig. 19-3, p. 379).
If an expelled complete gestational sac is not identified, sonography is performed to differentiate a complete abortion from threatened abortion or ectopic pregnancy. Characteristic findings of a complete abortion include a minimally thickened endometrium without a gestational sac. However, this does not guarantee a recent uterine pregnancy. Condous and associates (2005) described 152 women with heavy bleeding, an empty uterus with endometrial thickness < 15 mm, and a diagnosis of completed miscarriage. Six percent were subsequently proven to have an ectopic pregnancy. Thus, unless products of conception are seen or unless sonography confidently documents, at first an intrauterine pregnancy, and then later an empty cavity, a complete abortion cannot be surely diagnosed. In unclear settings, serial serum hCG measurements aid clarification. With complete abortion, these levels drop quickly (Connolly, 2013).
Missed Abortion
Also termed early pregnancy failure or loss, missed abortion, as originally defined, is contemporaneously misused compared with its meaning many decades ago. Historically, the term was used to describe dead products of conception that were retained for days, weeks, or even months in the uterus with a closed cervical os. Early pregnancy appeared to be normal with amenorrhea, nausea and vomiting, breast changes, and uterine growth. Because suspected fetal death could not be confirmed, expectant management was the sole option, and spontaneous miscarriage would eventually ensue. And because the time of fetal death could not be determined clinically, pregnancy duration—and thus fetal age—was erroneously calculated from the last menses. To elucidate these disparities, Streeter (1930) studied aborted fetuses and reported that the mean death-to-abortion interval was approximately 6 weeks.
This historical description of missed abortion is in contrast to that defined currently based on results of serial serum β-hCG assays and transvaginal sonography (Fig. 18-4). With rapid confirmation of fetal or embryonic death, many women choose uterine evacuation. Although many classify these as a missed abortion, the term is used interchangeably with early pregnancy loss or wastage (Silver, 2011).
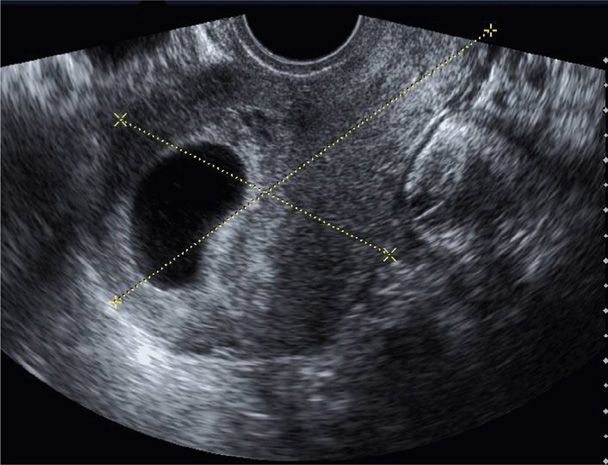
FIGURE 18-4 Transvaginal sonogram displays a large anechoic sac consistent with an anembryonic gestation. Calipers measure uterine length and anteroposterior thickness in a sagittal plane.
Septic Abortion
Horrific infections and maternal deaths associated with criminal septic abortions have become rare with legalized abortion. Still, perhaps 1 to 2 percent of women with threatened or incomplete miscarriage develop a pelvic infection and sepsis syndrome. Elective abortion, either surgical or medical, is also occasionally complicated by severe and even fatal infections (Barrett, 2002; Ho, 2009). Bacteria gain uterine entry and colonize dead conception products. Organisms may invade myometrial tissues and extend to cause parametritis, peritonitis, septicemia, and, rarely, endocarditis (Vartian, 1991). Particularly worrisome are severe necrotizing infections and toxic shock syndrome caused by group A streptococcus—S pyogenes (Daif, 2009).
During the last few years, rare but severe infections with otherwise low-virulence organisms have complicated medical abortions. Deaths have been reported from toxic shock syndrome due to Clostridium perfringens (Centers for Disease Control and Prevention, 2005). Similar infections are caused by Clostridium sordellii and have clinical manifestations that begin within a few days after an abortion. Women may be afebrile when first seen with severe endothelial injury, capillary leakage, hemoconcentration, hypotension, and a profound leukocytosis (Cohen, 2007; Fischer, 2005; Ho, 2009). Maternal deaths from these clostridial species approximate 0.58 per 100,000 medical abortions (Meites, 2010).
Management of clinical infection includes prompt administration of broad-spectrum antibiotics as discussed in Chapter 37 (p. 685). If there are retained products or fragments, then suction curettage is also performed. Most women respond to this treatment within 1 to 2 days, and they are discharged when afebrile. Follow-up oral antibiotic treatment is likely unnecessary (Savaris, 2011). In a very few women, severe sepsis syndrome causes acute respiratory distress syndrome, acute kidney injury, or disseminated intravascular coagulopathy. In these cases, intensive supportive care is essential (Chap. 47, p. 940).
To prevent postabortal sepsis, prophylactic antibiotics are given at the time of induced abortion or spontaneous abortion that requires medical or surgical intervention. The American College of Obstetricians and Gynecologists (2011b) recommends doxycycline, 100 mg orally 1 hr before and then 200 mg orally after a surgical evacuation. At Planned Parenthood clinics, for medical abortion, doxycycline 100 mg is taken orally daily for 7 days and begins with abortifacient administration (Fjerstad, 2009b).
 Management of Spontaneous Abortion
Management of Spontaneous Abortion
With embryofetal death now easy to verify with current sonographic technology, management can be more individualized. Unless there is serious bleeding or infection with an incomplete abortion, any of three options are reasonable—expectant, medical, or surgical management. Each has its own risks and benefits—for example, the first two are associated with unpredictable bleeding, and some women will undergo unscheduled curettage. Also, success of any method depends on whether the woman has an incomplete or missed abortion. Some of the risks and benefits are summarized as follows:
1. Expectant management of spontaneous incomplete abortion has failure rates as high as 50 percent.
2. Medical therapy with prostaglandin E1 (PGE1) has varying failure rates of 5 to 40 percent. In 1100 women with suspected first-trimester abortion, 81 percent had a spontaneous resolution (Luise, 2002).
3. Curettage usually results in a quick resolution that is 95- to 100-percent successful. It is invasive and not necessary for all women.
It is possible that patients and clinicians opt for surgical methods when there is not a strict protocol for medical treatment (Kollitz, 2011).
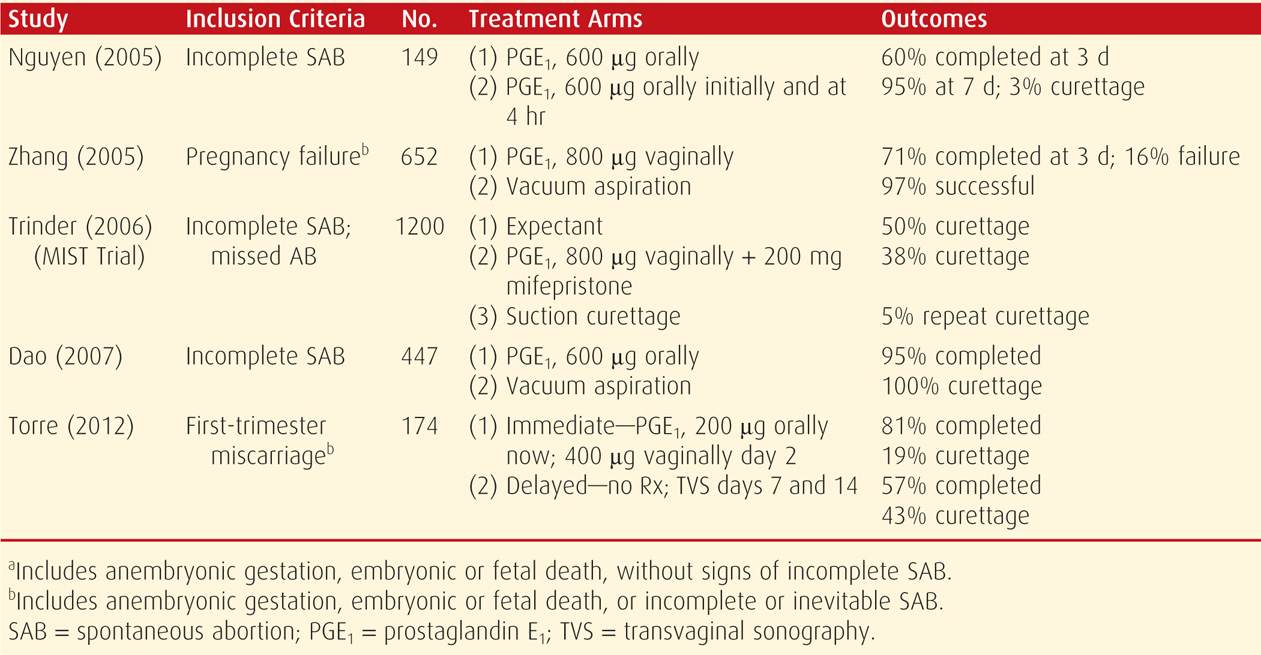
Several randomized studies that compared these management schemes were reviewed by Neilson (2010). A major drawback cited for between-study comparisons was varied inclusion criteria and techniques. For example, studies that included women with vaginal bleeding reported greater success for medical therapy than did studies that excluded such women (Creinin, 2006). With these caveats in mind, selected studies reported since 2005 are listed in Table 18-3. Importantly, Smith and coworkers (2009) reported that subsequent pregnancy rates did not differ among these management methods.
TABLE 18-3. Randomized Controlled Studies for Management of Various Types of First-Trimester Pregnancy Loss
RECURRENT MISCARRIAGE
Other terms that have been used to describe repetitive early spontaneous pregnancy losses include recurrent spontaneous abortion, recurrent pregnancy loss, and habitual abortion. It is generally accepted that approximately 1 percent of fertile couples have recurrent miscarriages classically defined as three or more consecutive pregnancy losses at ≤ 20 weeks or with a fetal weight < 500 grams. Most of these are embryonic or early losses, and the remainder either are anembryonic or occur after 14 weeks. Studies are difficult to compare because of nonstandardized definitions. For example, some investigators include women with two instead of three consecutive losses, and yet others include women with three nonconsecutive losses. Documentation of pregnancy with β-hCG levels, sonography, and pathological examination also varies widely.
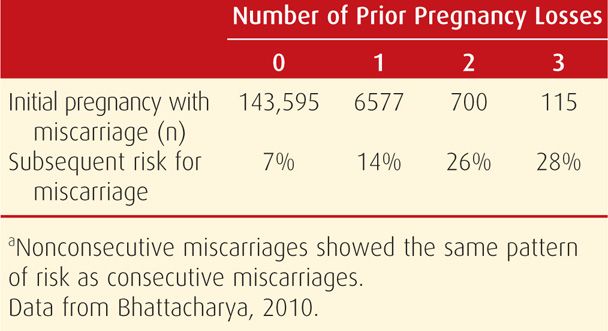
At minimum, recurrent miscarriage should be distinguished from sporadic pregnancy loss that implies intervening pregnancies that reached viability. Although women in the latter category were thought to have a much lower risk of yet another abortion, there are reports such as the one shown in Table 18-4 that question this assumption. In two studies, the risk for subsequent miscarriage is similar following either two or three pregnancy losses. Remarkably, the chances for a successful pregnancy are > 50 percent even after five losses (Brigham, 1999).
TABLE 18-4. Predicted Miscarriage Rate in Scottish Women with Their Next Pregnancy According to Number of Prior Miscarriagesa
The American Society for Reproductive Medicine (2008) proposed that recurrent pregnancy loss be defined as two or more failed clinical pregnancies confirmed by either sonographic or histopathological examination. A thorough evaluation certainly is warranted after three losses, and treatment is initiated earlier in couples with concordant subfertility (Jaslow, 2010; Reddy, 2007). Treatment considerations are beyond the scope of this book. The reader is referred to Chapters 6 and 20 in the 2nd edition of Williams Gynecology (Cunningham, 2012; Doody, 2012).
 Etiology
Etiology
There are many putative causes of recurrent abortion, however, only three are widely accepted: parental chromosomal abnormalities, antiphospholipid antibody syndrome, and a subset of uterine abnormalities. Other suspected but not proven causes are alloimmunity, endocrinopathies, environmental toxins, and various infections. Infections seldom cause even sporadic loss. Thus, most are unlikely to cause recurrent miscarriage, especially since maternal antibodies usually have developed. For years, various inherited thrombophilia mutations that include factor V Leiden, prothrombin G20210A, protein C and S deficiency, and antithrombin deficiency were suspected. But, as discussed in Chapter 52 (p. 1029), large studies have refuted an association between increased pregnancy wastage and these thrombophilias (American College of Obstetricians and Gynecologists, 2013a).
There is some evidence to support a role for various polymorphisms of gene expression in miscarriages. Just a few examples include polymorphisms that alter VEGF-A expression, those that exaggerate platelet aggregation, and those with a specific maternal type of Th1 and Th2 immune response (Calleja-Agius, 2011; Corardetti, 2013; Eller, 2011; Flood, 2010).
The timing of recurrent loss may offer clues, and in some women, each miscarriage may occur near the same gestational age (Heuser, 2010). Genetic factors usually result in early embryonic losses, whereas autoimmune or uterine anatomical abnormalities more likely cause second-trimester losses (Schust, 2002). As mentioned, first-trimester losses in recurrent miscarriage have a significantly lower incidence of genetic abnormalities than sporadic losses—25 versus 50 percent (Sullivan, 2004). That said, routine chromosomal evaluation of abortuses is costly and may not accurately reflect the fetal karyotype.
 Parental Chromosomal Abnormalities
Parental Chromosomal Abnormalities
Although these account for only 2 to 4 percent of recurrent losses, karyotypic evaluation of both parents is considered by many to be a critical part of evaluation. In an earlier study, balanced reciprocal translocations accounted for half of chromosomal abnormalities, robertsonian translocations for a fourth, and X chromosome mosaicism—47,XXY or Klinefelter syndrome—for 12 percent (Therapel, 1985). These chromosomal abnormalities are repetitive for consecutive losses (van den Boogaard, 2010). Inheritance of translocation syndromes and their sequelae are discussed in detail in Chapter 13 (p. 266).
After thorough genetic counseling, couples with an abnormal karyotype can usually be managed with IVF followed by preimplantation genetic diagnosis. These techniques are described in detail in Chapter 20 of Williams Gynecology (Doody, 2012).
 Anatomical Factors
Anatomical Factors
Several genital tract abnormalities have been implicated in recurrent miscarriage and other adverse pregnancy outcomes, but not infertility (Reichman, 2010). According to Devi Wold and colleagues (2006), 15 percent of women with three or more consecutive miscarriages will be found to have a congenital or acquired uterine anomaly.
Of acquired abnormalities, uterine synechiae—Asherman syndrome—usually result from destruction of large areas of endometrium. This can follow uterine curettage or ablative procedures. Characteristic multiple filling defects are seen with hysterosalpingography or saline-infusion sonography. Treatment is done using directed hysteroscopic lysis of adhesions. In many women, this lowers miscarriage rates and improves the “take home” pregnancy rate (Al-Inany, 2001; Goldenberg, 1995).
Uterine leiomyomas are found in a large proportion of adult women and can cause miscarriage, especially if located near the placental implantation site. That said, data indicating them to be a significant cause of recurrent pregnancy loss are not convincing (Saravelos, 2011). Uterine cavity distortion is apparently not requisite for bad outcomes (Sunkara, 2010). But in women undergoing IVF, pregnancy outcomes were adversely affected by submucous but not subserosal or intramural leiomyomas (Jun, 2001; Ramzy, 1998). As discussed in Chapter 63 (p. 1226), most agree that consideration be given to excision of submucosal and intracavitary leiomyomas in women with recurrent losses. Ironically, women undergoing uterine artery embolization of myomas had an increased risk for miscarriage in a subsequent pregnancy (Homer, 2010).
In contrast, congenital genital tract anomalies commonly originate from abnormal müllerian duct formation or abnormal fusion. These have an overall incidence of approximately 1 in 200 women (Nahum, 1998). The distribution of anomalies and associated loss rates are shown in Table 18-5. Depending on their anatomy, some may increase risks for early miscarriage, whereas others may cause midtrimester abortion or preterm delivery. Unicornuate, bicornuate, and septate uteri are associated with all three types of loss (Reichman, 2010). Looked at another way, developmental uterine anomalies were found in approximately 20 percent of women with recurrent pregnancy losses compared with about 7 percent of controls (Salim, 2003).
TABLE 18-5. Estimated Prevalence and Pregnancy Loss Rate for Some Congenital Uterine Malformations
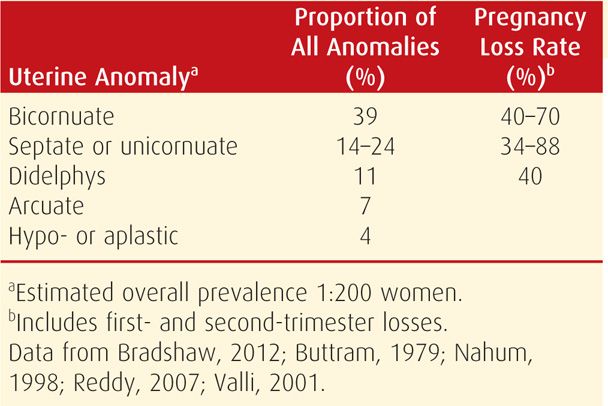
It has proven difficult to demonstrate that correction of uterine anomalies improves early pregnancy outcome. Additional discussion regarding the incidence, clinical impact, and treatment of anatomical abnormalities is found in Chapter 3 (p. 38), as well as in Chapter 18 of Williams Gynecology (Bradshaw, 2012).
 Immunological Factors
Immunological Factors
In their analysis of published studies, Yetman and Kutteh (1996) determined that 15 percent of more than 1000 women with recurrent miscarriage had recognized autoimmune factors. Two primary pathophysiological models are the autoimmune theory—immunity directed against self, and the alloimmune theory—immunity against another person.
As discussed on page 352, miscarriages are more common in women with systemic lupus erythematosus, an autoimmune disease (Clowse, 2008; Warren, 2004). Many of these women were found to have antiphospholipid antibodies, a family of autoantibodies that bind to phospholipid-binding plasma proteins (Erkan, 2011). Women with recurrent spontaneous pregnancy loss have a higher frequency of these antibodies compared with normal controls—5 to 15 versus 2 to 5 percent, respectively (Branch, 2010). The antiphospholipid antibody syndrome (APS) is defined by these antibodies found together with various forms of reproductive losses along with substantively increased risks for venous thromboembolism (American College of Obstetricians and Gynecologists, 2011d, 2013a). Mechanisms that cause pregnancy loss are discussed along with treatment in Chapter 59 (p. 1173).
With regard to alloimmunity, a provocative theory suggests that normal pregnancy requires formation of blocking factors that prevent maternal rejection of foreign fetal antigens that are paternally derived (Chap. 5, p. 98). Factors said to prevent this include human leukocyte antigen (HLA) similarity with the father, altered natural killer cell activity, regulatory T cell stimulation, and HLA-G gene mutations (Berger, 2010; Williams, 2012). Various tests and treatment options proposed to address this have not withstood rigorous scrutiny, and they are currently investigational (Reddy, 2007). Proposed therapies using paternal or third-party leukocyte immunization or intravenous immunoglobulin (IVIG) have not proved beneficial in women with idiopathic miscarriage (American Society for Reproductive Medicine, 2006; Stephenson, 2010).
 Endocrine Factors
Endocrine Factors
According to Arredondo and Noble (2006), 8 to 12 percent of recurrent miscarriages are caused by endocrine factors. Studies to evaluate these have been inconsistent and generally underpowered. Two examples, both controversial, are progesterone deficiency caused by a luteal-phase defect and polycystic ovarian syndrome (Bukulmez, 2004; Cocksedge, 2008; Nawaz, 2010).
In contrast, the well-known abortifacient effects of uncontrolled diabetes are detailed in Chapter 57. Optimal periconceptional glycemic control will mitigate much of this loss.
Likewise, the effects on early pregnancy loss of overt hypothyroidism and severe iodine deficiency are well known and discussed on page 353. Correction with supplementation reverses these effects. Also, the effects of subclinical hypothyroidism and antithyroid antibodies are sporadic, and thus any effects on recurrent miscarriage rates have been debated (Garber, 2012). That said, however, two recent metaanalyses reported convincingly positive associations between these antibodies and an increased risk for sporadic and recurrent miscarriages (Chen, 2011; Thangaratinam, 2011). Less convincing are preliminary data regarding thyroid hormone treatment for antibody-positive women.
MIDTRIMESTER ABORTION
The timespan that defines a midtrimester fetal loss extends from the end of the first trimester until the fetus weighs ≥ 500 g or gestational age reaches 20 weeks. For reasons discussed on page 350, a gestational age of 22 to 23 weeks is more accurate. Importantly, in many of these losses, an etiology can be found if a careful evaluation is completed.
 Incidence and Etiology
Incidence and Etiology
Abortion becomes much less common by the end of the first trimester, and its incidence decreases successively thereafter. Overall, spontaneous loss in the second trimester is estimated at 1.5 to 3 percent, and after 16 weeks, it is only 1 percent (Simpson, 2007; Wyatt, 2005). First-trimester bleeding doubles the incidence of second-trimester loss (Hasan, 2009; Velez Edwards, 2012). Unlike earlier miscarriages that frequently are caused by chromosomal aneuploidies, these later fetal losses are due to a multitude of causes and more closely reflect those discussed in the section under Recurrent Miscarriage (p. 358). There are no data to accurately estimate the incidences of these various causes, but some of the more common etiologies are listed in Table 18-6. One frequently overlooked factor is that many second-trimester abortions are medically induced because of fetal abnormalities detected by prenatal screening programs for chromosome trisomies and structural defects.
Risk factors for second-trimester abortion include race, ethnicity, prior poor obstetrical outcomes, and extremes of maternal age. First-trimester bleeding was cited previously as a potent risk factor (Hasan, 2009). Edlow and colleagues (2007) observed that 27 percent of women with such a loss in the index pregnancy had a recurrent second-trimester loss in their next pregnancy. Moreover, a third of these women had a subsequent preterm birth.
TABLE 18-6. Some Causes of Midtrimester Spontaneous Pregnancy Losses
Fetal anomalies
Chromosomal
Structural
Uterine defects
Congenital
Leiomyomas
Incompetent cervix
Placental causes
Abruption, previa
Defective spiral artery transformation
Chorioamnionitis
Maternal disorders
Autoimmune
Infections
Metabolic
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


