Materials and Methods
This is a retrospective case series of patients diagnosed with cesarean scar pregnancy or cervical pregnancy, between 6 and 8 weeks’ gestations, referred to New York University Langone Medical Center with diagnosed or suspected cesarean scar pregnancy and cervical pregnancy. This study was institutional review board approved (study number s15-01030 by the New York University Review Board).
Preliminary measurement of the inflated double-balloon catheter
To exert the right amount of pressure to stop embryonic cardiac activity to prevent bleeding and balloon expulsion, in vitro experiments were performed prior to the actual use of the double-balloon catheter (Cook Medical; www.Cookmedical.com ; number J-CRBS 18400 with stylet). By inflating the upper and lower balloon with increasing volumes of saline, the medical balloon sizes and the interballoon distance was measured. Figure 1 depicts the catheter and technique of selected experiments.
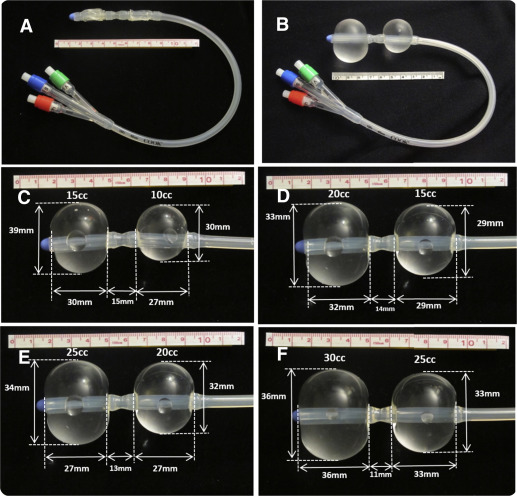
These measurements show that the upper, intrauterine balloon should be inflated with 30 mL or less fluid. The lower-treatment balloon should be inflated in the cervical canal or close to the internal os with no more than 20 mL fluid. Measurements at the actual use of the catheter were also performed to validate the previously mentioned in vitro measurements.
Diagnostic criteria for cesarean scar pregnancy and cervical pregnancy
In the presence of a positive pregnancy test and in patients with history of previous cesarean delivery, the criteria for a cesarean scar pregnancy were, as published earlier, the gestational sac and/or placenta were imaged embedded in the hysterotomy scar with a fetal pole and/or yolk sac containing a live embryo; empty uterine cavity and cervical canal; a thin (<3 mm) myometrial layer between the gestational sac/placenta and bladder and the presence of a rich vascular pattern in the area of the cesarean delivery scar and the placenta.
In patients without a previous cesarean delivery, a gestational sac and placenta seen within the anterior or posterior lip of the cervix, with a live embryo and/or yolk sac, and the presence of a rich vascular pattern around the sac were diagnostic for a cervical pregnancy.
The inclusion criteria
All patients who fulfilled the diagnostic criteria and consented to the double-balloon treatment after an evidence-based counseling were included in this study. The diagnosis, therapy, and follow-up of all patients were performed at the New York University Obstetrical and Gynecological Ultrasound Unit.
Inclusion criteria were gestational age between 6 and 8 weeks 6 days; demonstrable embryonic/fetal heart activity at the time of the ultrasound; a clearly stated desire for termination after evidence-based counseling describing the options for continuing or terminating the pregnancy; and signing an informed consent describing 2 treatment options of either local, intragestational methotrexate injection or the double-balloon technique described in the following text.
Description of the double-balloon–based treatment
Oral, nonsteroidal antiinflammatory pain medication was administered 2 hours before the procedure and continued as needed. Patients were prescribed a 5 day course of antibiotic treatment to be started on the day of treatment.
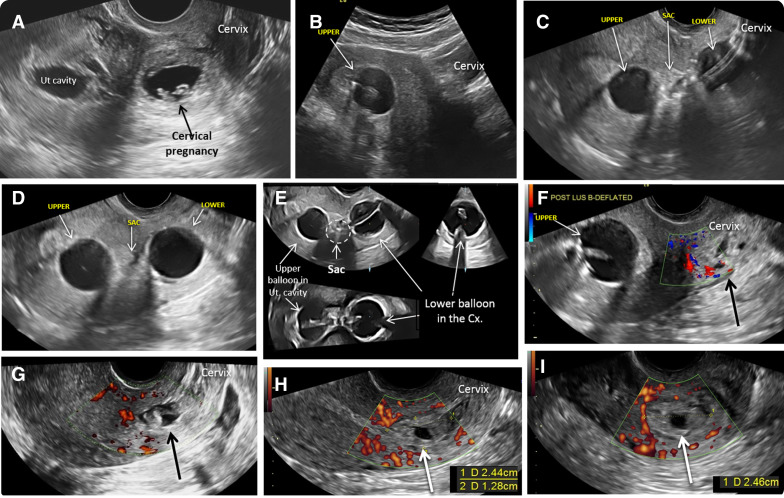
The patients were placed in lithotomy position. The vulva and vagina were prepped in a sterile fashion with betadine. An open-sided speculum was inserted, and the exposed cervix was cleaned with betadine. The size of the external cervical os was evaluated to fit the diameter of the catheter. If necessary, particularly in patients without prior vaginal delivery and/or no history of dilation and curettage, paracervical block (1% lidocaine) was administered followed by gently dilating the cervix to the size of Hegar number 7 to facilitate catheter placement. The uterus is imaged by a transabdominal ultrasound probe ( Figure 2 , A and B ). The sterile gel-lubricated, double-balloon catheter was advanced into the uterine cavity under continuous, real time transabdominal ultrasound guidance using sponge forceps.
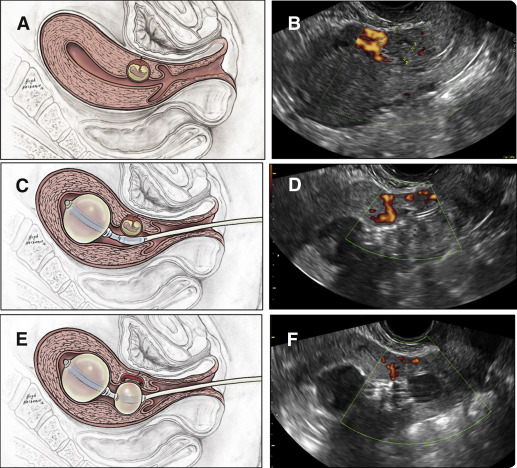
Under ultrasound guidance, the upper anchor balloon was inflated with 10 mL sterile saline to secure its position sonographically documented inside the uterine cavity ( Figure 2 , B and C). The speculum was removed and replaced by the transvaginal ultrasound probe. Under real time and continuous ultrasound observation, the lower-treatment balloon was positioned adjacent to the gestational sac. If needed, its position was readjusted inflating or deflating the anchoring upper balloon. The lower-treatment balloon was inflated by empirically adding saline until the gestational sac was flattened. The correct position of the balloon was sonographically documented ( Figure 2 , D and E).
The process of catheter placement and inflation of the balloons as well as the removal of the catheter is demonstrated in the attached video clip .
The area of the gestational sac and the lower balloon were observed by ultrasound, and if needed, saline was added to the balloons to prevent or stop any possible bleeding. Figures 3 and 4 present sequential and relevant ultrasound pictures obtained during treatments of a patient with cesarean scar pregnancy and a cervical pregnancy, respectively.
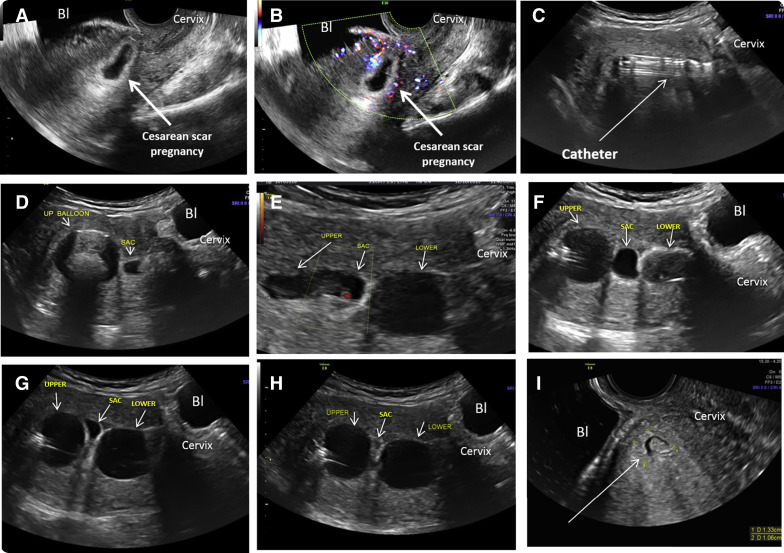
The patient was kept in the office under a nurse’s observation for 1 hour after which the uterus was rescanned transabdominally. If no heart beats were seen and there was no sonographic or clinical evidence of bleeding, the patient was discharged with instructions to return 2-3 days later for evaluation and removal of the catheter. An emergency day and night cell phone number and a printed report describing the procedure was given to the patient, should an emergency room visit be necessary.
At the return visit, the lower balloon was first deflated under transvaginal ultrasound control. If no heart activity and no visible bleeding was seen, the patient was observed by the nurse for 1 hour and then rescanned. If no local bleeding was noted, the upper balloon was deflated. If within an additional 30 minutes no change was detected, the catheter was removed and the patient discharged home with detailed instructions for scheduled repeat blood tests and ultrasound examinations.
Follow-up evaluation outcome
The patient’s follow-up consisted of weekly ultrasound examinations until the area of the sac demonstrated diminished vascularity as judged subjectively by the primary study investigators and until the gestational sac volume became smaller. Weekly serum human chorionic gonadotropin were obtained until nonpregnant values were noted. Birth control for 6 months was strongly suggested.
Statistical analysis
Statistical analyses were performed with IBM SPSS Statistics 22 (IBM Corp, Armonk, NY). Descriptive statistics (means and range) were calculated for: gestational age, sac volume, and serum human chorionic gonadotropin at treatment, days the balloon was kept in place, and days until human chorionic gonadotropin returned to nonpregnant values. Serum human chorionic gonadotropin and gestational sac volumes were analyzed over time.
Results
During the study period, 12 patients were diagnosed with cesarean scar pregnancy and cervical pregnancy with live embryos at the time of the treatment. After counseling, 2 patients with cesarean scar pregnancy preferred intragestational injection of methotrexate, and the double balloon was placed only for bleeding control. Thus, these 2 patients were excluded from statistical analysis. Ten patients (7 cesarean scar pregnancy and 3 cervical pregnancy) were treated by cervical double-balloon treatment and therefore were eligible for analysis.
Median gestational age was 6 6/7 weeks (range, between 6 3/7 and 7 4/7 weeks). Median gestational sac volume at treatment was 8.9 mL (range 2.5–25.8 mL). Median upper, anchor balloon was inflated with 24.0 mL saline (range 10–30 mL), whereas that of the lower, treatment balloon was 15.0 mL (range 8–21mL).
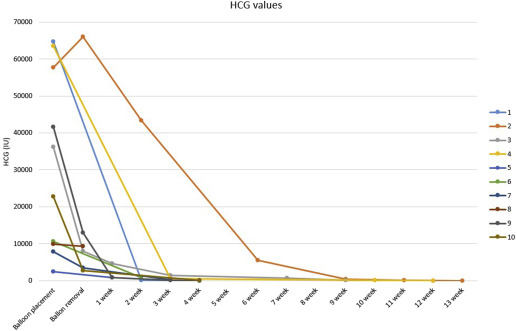
Balloons were kept in place for a median of 3 days (range, 1–5 days). Median serum human chorionic gonadotropin at the insertion of the balloons was 29,475 mIU/mL (range, 2488–64,700 mIU/mL). The median time for the human chorionic gonadotropin values to return to nonpregnant levels was 49 days (range, 28–97 days). In Figures 5 and 6 , the serum human chorionic gonadotropin and the gestational sac volume are documented as a function of time.