Introduction 32
Historical perspective 33
Muscles 42
The effects of exercise 45
Introduction
The newborn infant’s skeleton consists of largely cartilaginous ‘bone’ which is altered by external stresses over time. These forces and stresses are encountered in utero and continue, with the addition of direct gravity, postnatally.
One extreme example of the plasticity of the developing human foot is illustrated by the former traditional practice of binding the feet of baby girls in China. It was thought that the smaller the girl’s foot, the greater her marriage prospects and the greater the dowry – with no account being taken of how painfully crippled she might be. This process is described in hideous detail in Jung Chang’s memoir Wild Swans (Chang 1991).
Less distressing and more commonly encountered forces which affect the infant skeleton arise from:
• intrauterine position
• sleeping positions
• sitting postures.
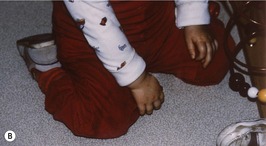
All can cause significant problems in the development and growth of the paediatric lower limb (Fig. 3.1A, B).
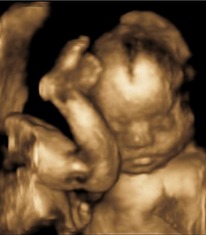
Twins and all multiple births are more susceptible to forces which can mould soft tissues and young bones. Hip instability, increased bone shaft torsions and metatarsus adductus have all been related to shared, and hence relatively reduced, intrauterine space (Fig. 3.2).
Historical perspective
In the 1940s, it became clinically evident that the growing skeleton would respond to modelling influences when deformities secondary to poliomyelitis were successfully treated and prevented using braces, splints and surgical transfers of muscle tendons. With the work of Sabin and Salk, poliomyelitis has been successfully vaccinated against and has dramatically declined in the developed world but it is still seen in children in poorer developing countries. The skills in managing lower limb deformities have now been adopted in the management of cerebral palsy, which, with the advent of amniocentesis and abortion choice, has also declined in prevalence.
Two decades later, in the 1960s, it was found that the abnormal forces produced from spastic muscle contractions caused the deforming of structures being produced from normal bone development. Previously it had been thought that the bony deformities in spastic children were due to abnormal cartilage/bone development. This was quite a step forward in understanding and redirected treatment approaches to be more functional in addition to the use of passive splinting.
Anatomical overview
The lower limb consists skeletally of:
• long bones
• short bones
• sesamoid bones
• accessory bones, if present.
Long bones are tubular (e.g. femur, tibia, fibula, metatarsals, phalanges) while short bones are more cuboidal (e.g. the tarsal bones). Sesamoid bones develop within particular tendons (e.g. patella) and are located at the junction of tendons crossing the ends of long bones (e.g. flexor hallucis brevis and first metatarsal) and function in assisting tendon leverage. Accessory or ‘supernumerary’ bones develop when extra ossification centres appear and form additional bones (e.g. navicular and os tibiale externum which has approximately 10% incidence).
Heterotopic bones are unusual and form within soft tissues (e.g. scars) as a result of calcification of small muscular haemorrhages (may be referred to as ‘rider’s bones’ when found in the adductors of horse riders; Moore, Dalley, 1999 and Moore, Dalley, 1999).
All skeletal bones are derived from the embryonic mesenchyme either by direct intramembranous ossification or by endochondral ossification, i.e. via a cartilage template.
There is no difference in histology of bones formed by intramembranous ossification or endochondral ossification. As it is endochondral ossification which largely pertains to the lower limb, a brief outline is included below:
• Mesenchyme differentiates into chondroblasts.
• Chondroblasts form a cartilaginous bone model.
• Cartilaginous bone model calcifies centrally and capillaries infiltrate the interior of the bone model.
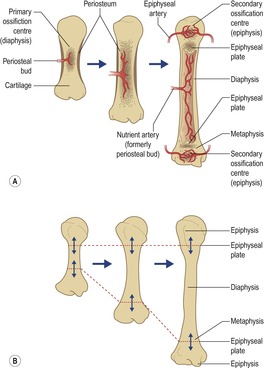
• Capillaries and osteogenic cells form a periosteal bud or primary ossification centre.
• Primary ossification centre becomes the diaphysis (Cusick 1990, Moore, Dalley, 1999 and Moore, Dalley, 1999).
Diaphysis
• Comprises the main cortical structure of a long bone.
• The process of endochondral ossification involves progressive destruction and replacement of the hyaline cartilage model.
• Many eventual skeletal deformities are due to chondrification error, after which normal bony modelling and ossification occur → abnormal skeletal structure.
Metaphyses
• Located at diaphyseal poles.
• Characterized by less cortical thickness and more trabecular bone.
• Prior to completion of mineralization, trabecular bone may be modelled by force.
• Very porous and highly vascular.
Epiphyses
• Secondary ossification centres, located at either end of the diaphysis (the only short bone to develop a secondary ossification centre is the calcaneus; Fig. 3.3).
• Totally cartilaginous at birth with the exception of the distal femur and proximal tibia.
• Epiphyseal arteries infiltrate as do osteogenic cells (e.g. osteoblasts).
• Throughout growth cartilage is progressively replaced by bone, leaving articular cartilage (hyaline) at skeletal maturation.
• Epiphyses with a tendon attached are termed apophyses and are predisposed to avulsion injury (e.g. distal fibula, base fifth metatarsal, anterior tibial tubercle).
Physis
• Bony growth plates which intervene between the diaphysis and epiphyses during long bone growth.
• Vulnerable to mechanical disruption, especially from shearing forces.
• Consists of several cellular layers embedded in ground substance.
• Growth ceases when the growth plates are completely replaced by bone fusing the epiphysis with the diaphysis. This usually occurs 1–2 years earlier in girls than boys for the same anatomical sites.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree