Fig. 23.1
Light microscopy of neuroglial hamartoma. a Mature neuroglial tissue with ribbons of ganglionic elements. b Immunohistochemically, ganglion cells with neuritic and axonal processes are strongly immunoreactive for S100 protein. c Nerve structure surrounded by concentrically arranged perineurial cells. d Neuroglial component immunoreactive for glial fibrillary acidic protein (GFAP)
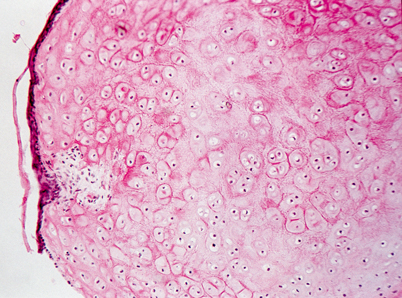
Fig. 23.2
Light microscopy of external ear canal hamartoma. Cartilaginous nodule partially covered by attenuated squamous epithelium
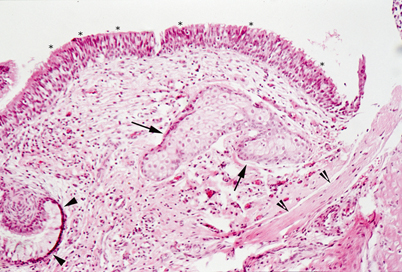
Fig. 23.3
Light microscopy of middle ear hamartoma. Connective tissue with sebaceous glands (arrows) and bundles of smooth muscle (clefted arrowheads) is covered by respiratory type ciliated epithelium (asterisks) and foci of mucous cells (arrowheads)
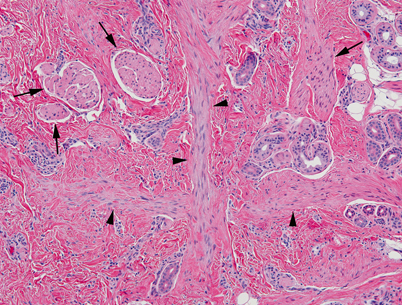
Fig. 23.4
Light microscopy of dermal hamartoma. Abnormal dermal tissue with enlarged erectory pili (arrows), thick bands of smooth muscle (arrowheads) and disorganized adnexa
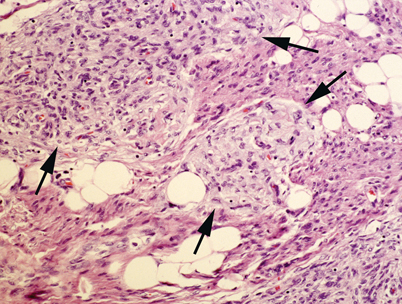
Fig. 23.5
Fibrous hamartoma of infancy. The lesion is composed of a mixture of mature adipose tissue, fibrocollagenous bands and islands of primitive mesenchyme (arrows). Fibrous hamartoma of infancy of the head and neck is usually located in the scalp
Biology and Epidemiology
Pathophysiology
The clinical presentation of hamartomas will depend upon the area of involvement.
Hypothalamic hamartomas (HH) are associated with seizure activity and/or central precocious puberty (CPP) .
Estimated that 14–58 % of CPP cases are caused by HH; HHs are the most common cause of CPP [2].
Gelastic seizures are common and notoriously intractable.
The hypothalamic hamartoma is intrinsically epileptogenic.
Molecular/Genetic Pathology
A molecular pathway to hamartoma formation can be seen in the disorder tuberous sclerosis, in which subependymal giant cell astrocytomas form [3]:
Caused by mutation in either of two tumor suppressor genes, TSC1 or TSC2.
The products may be involved in the inhibition of tumor formation.
The inheritance pattern of hamartomas is dependent upon the specific tumor and area of involvement.
Incidence and Prevalence
Pediatric hamartomas are rare .
Hamartomas are the second most common benign pediatric pulmonary tumor of the lung, though bronchial tumors in general are rare [11].
Age Distribution
Sex Predilection
Relationships to Other Disease States, Syndromes
Tuberous sclerosis may be associated with West Syndrome [3] .
Presentation
Presentation depends on area of involvement:
Stridor
Dysphagia
Dysphonia
Dyspnea
Earache
Aspiration
Nasal obstruction
Respiratory and feeding difficulties in infants
Epistaxis
Rhinorrhea
Serous otitis media
If orbital involvement: proptosis, enophthalmos, ophthalmoplegia, ptosis, or hypotropia
If intracranial expansion: hydrocephalus or with oculomotor disturbances
Figure 23.6 shows a gross example of a nasal mass.
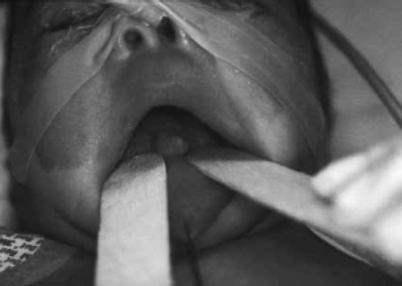
Fig. 23.6
An anterior, polypoid tongue mass that proved to be a hamartoma upon histological examination. (Reprinted from Horn et al. [8], with permission of SAGE Publications)
Tongue lesions [7]:
Airway obstruction
Oral bleeding
Cosmetic concern
Dysphagia
Dysarthria
Respiratory distress, especially if in posterior tongue
Figure 23.7 shows a gross example of a tongue lesion.
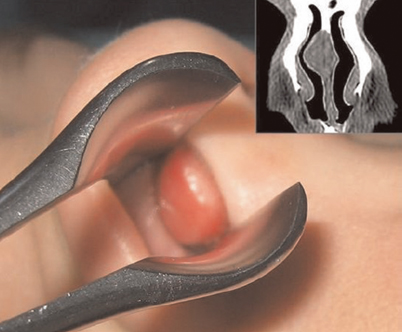
Fig. 23.7
A gross image of a nasal hamartoma that filled the right nasal meatus of a child, with the computed tomography (CT) scan revealing the right nasal mass adjacent to the nasal septum. (Reprinted from Gajda et al. [13])
Tracheal [9]:
Similar to intractable asthma or obstructive airway disease
Expiratory wheezing
Biphasic stridor
Solitary mobile and firm mass, gradually enlarging
Most are 2.5–5 cm in diameter
Can occur on neck
Occasional skin change, such as pigmentation changes or eccrine gland hyperplasia
Central precocious puberty
Seizures, typically gelastic
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


