CHAPTER 33 Wilms Tumor
Step 1: Surgical Anatomy
Normal Anatomy
♦ Although extrarenal Wilms tumor can occur, the vast majority of Wilms tumors occur in one or the other kidney. No side predilection has been observed.
Anatomic Variations
♦ Variations in renal anatomy can occur. It is critical to recognize certain ones, such as the occurrence of Wilms tumor in a solitary kidney or horseshoe kidney, because these variations will significantly alter the treatment approach.
♦ Variations in the renal vasculature are fairly common. These include multiple renal arteries or veins and a circumaortic or retroaortic left renal vein. However, these variants rarely impact the conduct of a radical nephrectomy and do not need to be exhaustively sought with preoperative imaging.
Step 2: Preoperative Considerations
Imaging
Assessment of the Primary Tumor
♦ Ultrasonography is often used as an initial screening study for an infant or young child with an abdominal mass.
♦ Computed tomography (CT) of the abdomen and pelvis is generally the imaging study of choice for patients suspected of having a renal tumor. CT will confirm the presence of a solid renal mass and will also afford the opportunity to visualize the contralateral kidney to confirm its presence (and function) and to exclude synchronous bilateral disease.
Assessment of Vascular Extension
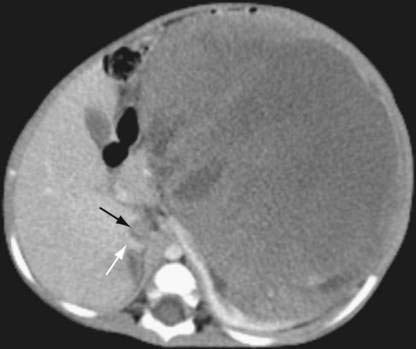
♦ Intravascular tumor extension into the inferior vena cava occurs in about 6% of Wilms tumor cases (Fig. 33-1). Therefore, this should be specifically investigated in the preoperative evaluation of all children with a renal mass because it might alter the conduct of surgery. This investigation can generally be done most easily and accurately using Doppler ultrasonography. Magnetic resonance imaging can also be used to assess intravascular tumor extension but usually requires sedation in young children and so is not routinely used. However, it may be helpful in defining an extensive tumor thrombus that extends up to the level of the hepatic veins or even into the right atrium. Echocardiography may be useful in rare circumstances to demonstrate (or exclude) intracardiac tumor extension.
Assessment of Metastatic Disease
♦ The most common site of metastatic spread of Wilms tumor is the lungs; chest CT is the preferred imaging modality for evaluating this site. However, the presence of a small pulmonary nodule is not always metastatic disease; histologic confirmation is required either before to the start of therapy or after administration of three-drug therapy, if the lesion(s) persists.
Associated Medical Conditions
Renal Function
♦ Patients with Wilms tumor rarely present with renal insufficiency, so formal preoperative assessment of renal function is usually not required.
♦ Patients with a Wilms tumor predisposition syndrome may have intrinsic renal disease, which might not be of clinical significance until a few years later. However, because of this, the Children’s Oncology Group currently suggests that children with a unilateral tumor and an associated Wilms tumor predisposition syndrome (defined below) be managed on a treatment protocol that includes unilateral nephron-sparing surgery.
Pulmonary Function
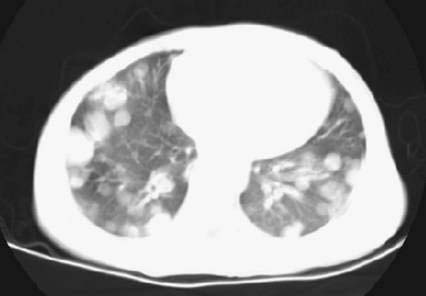
♦ Children with Wilms tumor occasionally present with pulmonary insufficiency from extensive metastatic disease in the lungs (Fig. 33-2).
♦ The clinical assessment is generally sufficient to determine whether the patient can tolerate general anesthesia; formal pulmonary function studies are not required.
♦ Patients who appear unable to tolerate general anesthesia should have a percutaneous biopsy, if feasible, and a central venous catheter placed so that three-drug neoadjuvant chemotherapy can be initiated. Because Wilms tumor is generally chemosensitive, the tumor burden in the lungs and the functional pulmonary status should improve fairly promptly.
Hypertension
♦ About 25% of children with Wilms tumor will present with hypertension. A preoperative assessment of blood pressure should be performed in all patients, with hypertension being medically controlled before surgery. In many patients, but not all, the hypertension will resolve after radical nephrectomy, although it may take some time.
Predisposition Syndrome
♦ Some patients (2%) will have a Wilms tumor as part of their presentation of a Wilms tumor predisposition syndrome (Table 33-1). Patients with “predisposition syndromes” include those with unilateral Wilms tumor and aniridia, Beckwith-Wiedemann syndrome, hemihypertrophy, Simpson-Golabi-Behmel syndrome, and Denys-Drash syndrome.
♦ These patients are currently being treated with neoadjuvant chemotherapy and unilateral nephron-sparing surgery. Appropriate genetic testing may be done as indicated.
Table 33-1 Wilms Tumor Predisposition Syndromes
| NAME | GENETIC ABNORMALITY | ASSOCIATED ANOMALIES |
|---|---|---|
| WAGR syndrome | 11p13 (WTI) deletion | Aniridia, Genitourinary anomalies, mental Retardation |
| Denys-Drash syndrome | WTI mutation | Nephropathy, intersex disorders |
| Beckwith-Wiedemann syndrome | 11p15 (WT2) abnormalities | Microsoma, macroglossia, visceromegaly, embryonal tumors, omphalocele, hypoglycemia |
| Sporadic hemihypertrophy | Unknown | Enlargement of one side of the body or part of the body |
| Simpson-Golabi-Behmel syndrome | X-linked recessive | General overgrowth in height and weight with characteristic facial features |
Step 3: Operative Steps
Incision
♦ A transabdominal approach to the affected kidney is currently recommended; a flank or laparoscopic approach is discouraged for performing a radical nephrectomy. A variety of incisions can be used based on the size of the lesion, the presence of intravascular tumor thrombus, and the preference of the operating surgeon. These include an upper abdominal/transverse incision, a subcostal incision, a midline incision, and a thoracoabdominal incision. The incision should be large enough that the integrity of the specimen is not compromised on removal from the abdominal cavity, as tumor rupture and spill have significant consequences.
Tumor/Kidney Removal
♦ A full visual and manual exploration of the peritoneal cavity, including the liver surfaces, should be performed, although this is often difficult to do until after the primary tumor specimen has been removed. The occurrence of preoperative rupture should be documented. Although early ligation and division of the renal vessels have been traditionally recommended, it appears that complete mobilization of the affected kidney and associated tumor first protects against inadvertent ligation of mesenteric vessels or even the aorta or inferior vena cava, and so is now recommended.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree