We are now going to describe, the principle cardiac pathologies that are encountered prenatally.
First step. pathologies of position
Pathologies of position are the first we look for when examining the fetal heart. Beginning the examination abdominally, we observe the following.
Anomalies of visceral positioning
In the case where the stomach and heart are not on the same side (Fig. 6.1), the anomaly is clear. More easily overlooked is the case where the stomach is positioned on the right of the fetus along with the heart, a situation which has the same pathologic relevance.
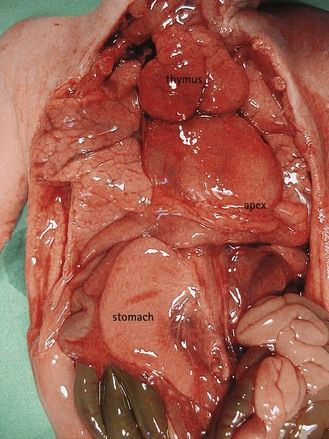
Figure 6.1 The apex of the heart towards the left and the stomach in the right side in a visceroatrial heterotaxia.
Situs anomalies often go unnoticed until the fetal pathologic or postnatal examinations.1 They are frequently associated with complex cardiopathies and only worsen the prognosis. These anomalies belong to the visceroatrial heterotaxias (VAH) group with risk of recurrence.
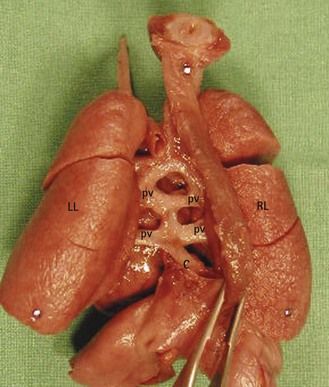
In VAH, gene lateralization anomalies2 encourage the formation of two homologous sides. Schematically, the fetus that presents with VAH, instead of having a normal left side and a normal right side has a “doubling,” which is neither really left nor right. These abdominal abnormalities are not systematic. They typically affect the liver which becomes median (Fig. 6.2) with the gall bladder situated to the right or left. The spleen, an organ normally found on the left, is classically absent (asplenia) in the case of a right isomerism, or multiple (polysplenia) in the case of a left isomerism. These anomalies are difficult to see with ultrasound (US), especially if we do not systematically verify the lateralization of the vessels and the organs at the abdominal level.
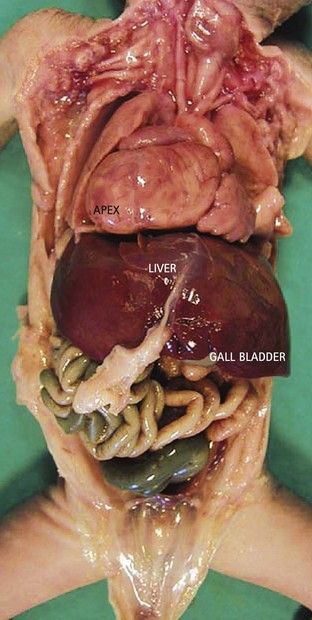
Figure 6.2 Median liver in a visceroatrial heterotaxia. Note on the right the apex of the heart, which is not normal.
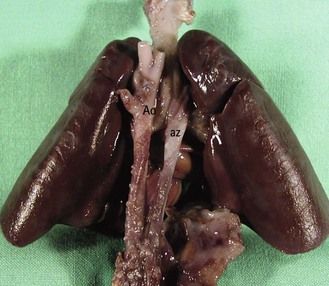
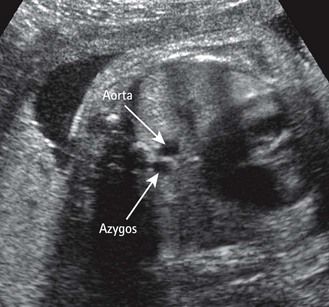
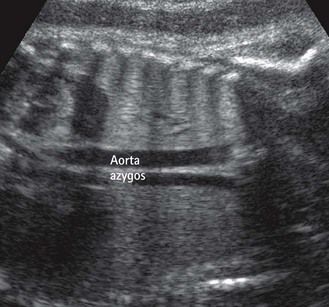
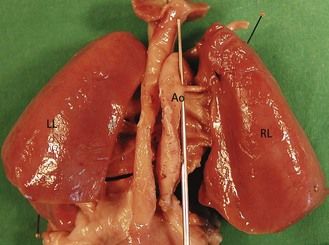
At the same time, prenatally, it is impossible to identify two atrial appendages of the same type that would define a right or left isomerism. But this type of atrial appendage “absence” is often accompanied by anomalies of venous return corresponding to the appendage that is “missing.” In left isomerism (two left-type appendages, and therefore no atrium of the right type) the anomaly is found in the systemic venous return that normally would occur in the “missing” right atrium (RA). This is abnormal and is the reason why we can observe an azygos return (Fig. 6.3) with absence of the suprarenal section of the inferior vena cava (IVC), an element which can be recognized in US3 by a transverse (Fig. 6.4) or longitudinal (Fig. 6.5) view. In addition, this absence of the RA—which is the normal center for the principal elements of the conduction system—can provoke rhythm problems. In a right isomerism, where the left appendage is missing, we need to consider searching for anomalies related to pulmonary venous return (PVRs), i.e., total or partial PVR.
Vessel position anomalies
In requiring verification of the vessels at the level of the TAD, following them through the “elevator”, then arriving at the four-chamber view, we can observe several possibilities.
Not one but two vessels in front and to the left of the spine on the TAD image
These are behind the LA in the four-chamber view (see Figs 6.4 and 6.5). This is the situation in an azygos venous return, classically observed in cases of left isomerism with the absence of a section of the IVC seen at the level of the TAD image.
Though not frequent, and yet of great interest, is the discovery of an infra-diaphragmatic confluent of a totally anomalous PVR (TAPR). When isolated, the TAPR can be seen to be recurrent within the same family. This has been observed in the form of a third abdominal vascular mass. It is part of the range of VAH syndromes, and often associated with other pathologic elements of this condition which can be far more difficult to diagnose.
Anomalies of organ or vessel position at the abdominal level, which are present in VAH, are elements of orientation
These are easier to diagnose than the frequent complex CHD associated with them. Often affecting the inlet and outlet, VAH cardiopathies found in the inlet are essentially represented by a unique atrium (UA) frequently associated with AVSD or unique ventricle (UV). In the outlet they are often seen as PA with OS, with or without the great vessels being transposed.8
To this end, the discovery of an AVSD in a patient having a normal karyotype should immediately lead us to consider VAH.9
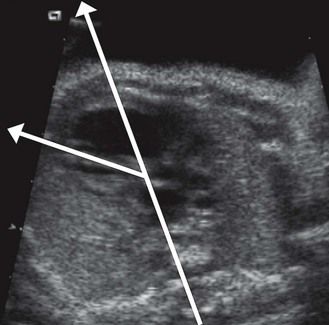
The descending aorta is found—not in front and to the left—but on the right of the spine in the four-chamber view
A right descending aorta after a right aortic arch facilitates our diagnosis when we remember to look for it systematically using the four-chamber view. It is an excellent warning sign of conotruncal cardiopathies (CTC) (Fig. 6.6),13 the most direct indications for which should only be seen at the outlet level (Fig. 6.7). In addition, this sign, when the standard karyotype is normal, points us towards 22q11 deletion (Fig. 6.8).
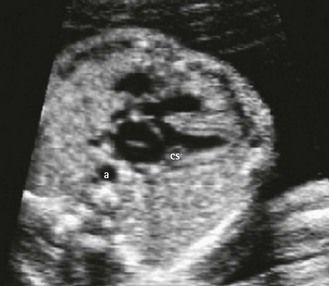
Figure 6.6 “Pediatric” four-chamber view showing a right descending aorta, the axis of the heart (at 60°), and the dilated coronary sinus. We can already see that the VSD predominates in ejection.
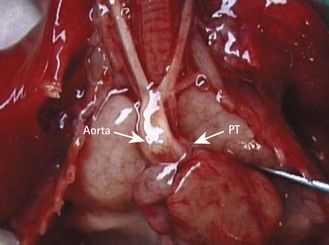
Figure 6.7 Tetralogy of Fallot in trisomy 21 with an arch and right descending aorta. Note the large aorta and the small PT.
Anomalies concerning the position of the heart
The heart is in the right hemithorax (Fig. 6.9), conserving its apex to the left. This case is often associated with a left diaphragmatic hernia (Fig. 6.10). Diaphragmatic hernias are particularly seen in forms of this pathology which are part of a general group of syndromes, (e.g., Fryn syndrome). Associated CHD can also exist here, which would also be generally conotruncal.
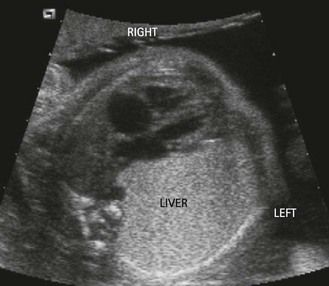
Figure 6.9 Ultrasound view showing the liver in the left hemithorax in a diaphragmatic hernia. The apex of the heart turns towards the left.
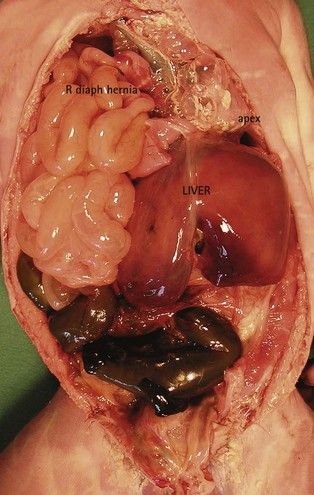
Figure 6.10 Macroscopic view showing liver and intestine in the right hemithorax in a diaphragmatic hernia. The apex of the heart turns towards the left.
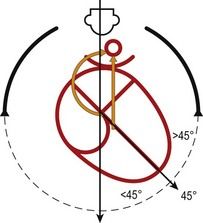
Anomalies that modify the axis of the heart
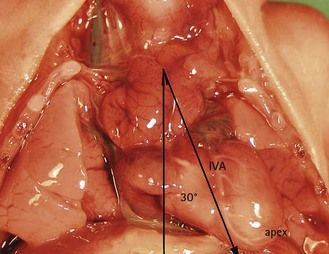
The interventricular septum (IVS), which marks the boundary between the right and left inlet tracts, represents the axis of the heart on the four-chamber view. It normally makes a medium-sized angle of 45° with the anteroposterior axis and reflects the balance between the inlet tracts. The right inlet tract is composed of the RA and the inlet section of the RV, while the left inlet tract is composed of the LA and the inlet section of the LV. Any modification of this axis should be observed by the operator during examination using the four-chamber view (Fig. 6.11). Pathologically it is perhaps the asymmetry of the chambers causing an important modification of the axis which attracts our attention.16
The angle can be clearly superior to 45 with a distinct asymmetry of the chambers
This is seen in severe hypoplasias of the left tract (Figs 6.12 and 6.13) as well as in Ebstein disease. Here (Fig. 6.14) the dilatation of the RA can be enormous due to an incompetent tricuspid valve. This is especially true due to the fact that the septal valve remains stuck to the septum much more than it normally would, enlarging the atrium, which then appears larger than the ventricle. Forms of this anomaly observed prenatally are often severe.
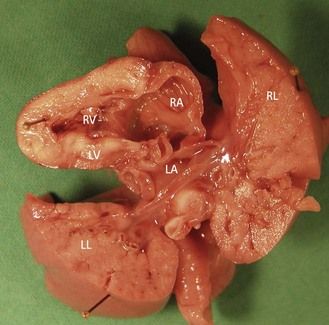
Figure 6.12 Macroscopic view where the axis is at an angle greater than 45° in a hypoplasic LV caused by mitral atresia.
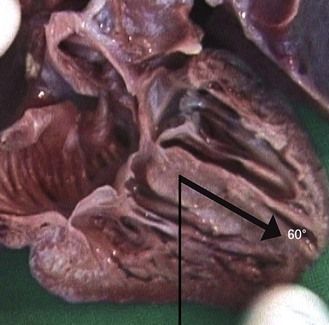
The inlet chambers remain symmetric
However, their angle can attain 60° in forms of tetralogy of Fallot (ToF) where the heart takes on the form of a “boot” due to the overriding of the aorta upon the VSD (Fig. 6.15; see also Fig. 6.5).
The angle can be inferior to 45°
This is the case in hypoplasia of the RV called pulmonary atresia with intact septum (PA with IS) (Figs 6.16 and 6.17) that is to say without VSD.17 Care should be taken not to confuse this condition with pulmonary atresia with open septum (PA with OS), which is a major form of CTC caused by the anterior swing of the conal septum and which has a constant VSD. In the case of PA with IS (exterior to the small size of the PT and RV) fistulae can exist within the coronary circulation on the wall of the RV which are visible on the RV wall in fetal pathology (Fig. 6.18) and translate by multiple aliasing on Doppler. Their hemodynamic consequence can bring about uterine death.18
The axis can be negative with the apex of the heart to the right
This could be due to a heart that is a mirror image with complete situs inversus (Fig. 6.19). While cardiac situs inversus is associated with an abdominal situs inversus, this anomaly, which falls in the category of VAH, often remains unnoticed. A dextrorotation due to an atrioventricular discordance with a normal atrial situs can also displace the apex towards the right.
Second step. pathologies of the inlet
To each key point, one or several pathologies corresponds:
Point 3: heart on the diaphragm
If we do not discipline ourselves to always use the inferior PVs as our reference point—and separate to the axial errors already described in lateral swings—we can miss an extremely serious anomaly concerning PVR. TAPVR is seen as either:
Point 4: if we cannot distinguish the four chambers
In this situation we can identify the following:
Three chambers
Three chambers in the case of a UA or UV. The diagnosis of UA, frequently associated with AVSD, should not pose a particular problem in the total absence of an interauricular wall without a flap valve. This should lead us to search for Ellis–van Creveld syndrome (Fig. 6.22).
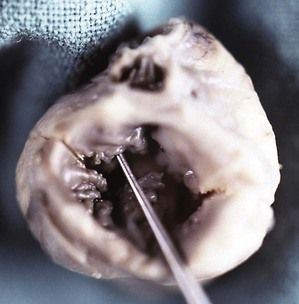
Figure 6.22 Right posterior view of the fetal heart with Ellis–van Creveld syndrome showing a UA overhanging a complete AVSD. Note the dysplasia of the bridging leaflets.
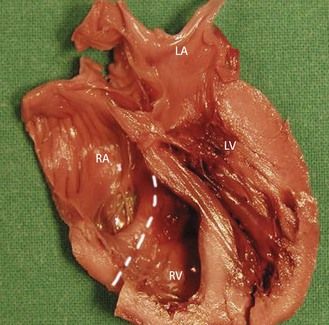
The case of a UV is more delicate because of the difficulty in differentiating a UV from an extreme ventricular hypoplasia, in particular that of the left. In this case, the papillary muscle of the RV, which is large, is often mistaken as the IVS. But in these cases, careful analysis of the four-chamber view will not allow us to find balanced chambers with a normal crux of the heart.
Four+ chambers
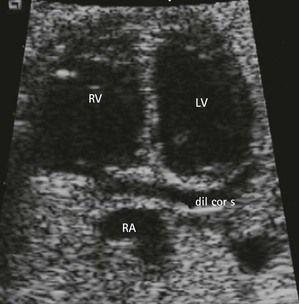
Seeing a small, supplementary, rounded chamber at the left atrioventricular angle (Fig. 6.23; see also Fig 6.6) is witness to a most often dilated coronary sinus (CS) by a persistent left subclavian vein (LSCV). The importance of this dilatation and its eventual repercussions22 depend on the association of the LSCV with an abnormal PVR found flowing there. Erroneous diagnoses of AVSD have been made in the case of a dilated CS in the presence of an imperfect four-chamber view (Fig. 6.24).23
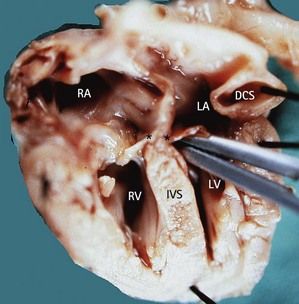
Figure 6.23 Macroscopic view of the four chambers with a dilated CS in trisomy 21. Note the LIAVV without defect (marked by *).
Five chambers
We should be aware of the existence of a very rare doubling of the left atrial chamber: the triatrial heart.24 This “antechamber” receives the PV and is in communication with the LA by a tight orifice, which brings about the same effect as that of mitral stenosis with eventual repercussions on the development of the left tracts.
Point 5: asymmetric or discordant chambers
If the chambers are asymmetric we can distinguish a variety of architectural malformations
Right ventricular hypoplasia due to PA with IS (see Fig. 6.18) is found more often than tricuspid atresia (Fig. 6.25). In tricuspid atresia—certain cases have been published showing this condition to be associated with 22q11 deletion25—the right AV valve is closed but, due to the persistence of the bulbar ventricular foramen ensuring communication between the left and right outlet, the great vessels can be balanced. They are, however, often transposed.
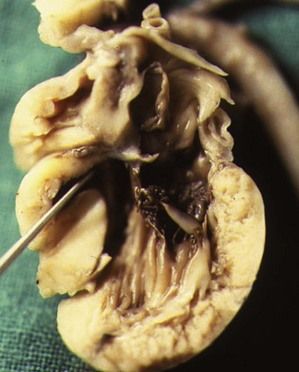
Figure 6.25 Tricuspid atresia seen within a macroscopic four-chamber view showing a dead-ended orifice.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree