CHAPTER 54 Voiding difficulty
Introduction
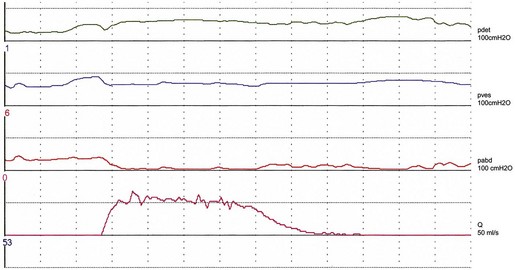
Studies on detrusor pressure during voiding in normal women suggest that most void with a detrusor contraction greater than 15 cmH2O (mean detrusor pressure at maximum flow 23–28 cmH2O, maximum detrusor pressure 20–36 cmH2O) (Figure 54.1). Detrusor pressure during voiding is generally lower in women than in men. A few normal women have been reported to void with a low pressure detrusor contraction of less than 15 cmH2O, but no normal women void with no contraction at all; such an event may indicate low urethral closure pressure.
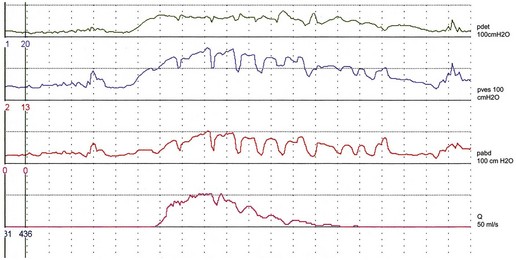
There are variations in the way that women void. Some women, in addition to pelvic relaxation and detrusor contraction, may strain by an unconscious but sustained Valsalva manoeuvre (Figure 54.2). The contribution of abdominal pressure to the voiding process varies considerably between individuals and within the same individual during consecutive voids.
The ‘normal’ voiding curve in women has a single peak, with a fast crescendo and a relatively slow diminuendo, with minimal fluctuations. However, an interrupted pattern can be seen repeatedly in a minority of normal women. Studies of flow rates suggest that most normal women void with a peak flow rate greater than 15 ml/s, with mean values of 23–27 ml/s (Figure 54.1). Peak flow rate values are generally higher in women than in men. The main variable affecting flow rates in normal women is bladder volume, with greater flow rates seen with increasing volumes. Parity, weight and height have no influence on flow rates.
Definitions
Voiding difficulty
A joint International Urogynecological Association/International Continence Society (ICS) Report on terminology for female pelvic floor dysfunction defines ‘voiding difficulty’ as ‘abnormally slow and/or incomplete micturition’ (Haylen et al 2010). The diagnosis is obtained by symptoms (see below) and urodynamic investigations, and should be based on repeated measurements to confirm abnormality.
Abnormally slow urine flow rates, as determined by uroflowmetry, are best referenced to nomogram charts which provide a range of normality for urinary flow rates in relation to volume voided; abnormal flow is defined as flow under the 10th centile (Haylen et al 1989). To encourage the use of these charts, a larger version of the original charts has been republished recently (Haylen et al 2008a).
Abnormally high postvoid residuals (i.e. volume left in the bladder at the completion of micturition) depend on the method used for measurement and the timing of the measurement after micturition, taking into account renal input of 1–14 ml urine/min (Haylen et al 2010).
Upper limits of postvoid residuals of 30 ml (using immediate ultrasound assessment) and 50–100 ml (using urethral catheterization) have been proposed (Haylen et al 2010).
Acute retention of urine
This is defined as a generally, but not always, painful, palpable or percussable bladder, when the patient is unable to pass any urine when the bladder is full (Haylen et al 2010).
Incidence
Depending on definition and type of clinic, voiding difficulty in women presenting to a urology or urogynaecology clinic has a variable prevalence ranging from 14% (when using a strict definition based on multiple variables including low flow, high pressure and increased postvoid residual) (Massey and Abrams 1988) to 39% (using a postvoid residual of 30 ml or more) (Haylen et al 2007).
Symptoms and Clinical Effects
Inability to void usually leads to prolonged use of urinary catheters and an increased incidence of UTI. The risk of acquiring bacteriuria relates to the duration of catheterization, and ranges from 4% to 7.5%/day over the first 10 days of catheterization (Schaeffer 1986). The majority of patients on long-term clean catheterization have bacteriuria, and about one-third of them require intermittent treatment with antibiotics due to symptomatic infection (Lapides et al 1976). Catheter-related UTI is an important cause of hospital-acquired infections, morbidity (e.g. pyelonephritis) and, in the elderly, mortality.
Causes
The causes of voiding difficulty are shown in Box 54.1.
Effects of age on voiding function
The incidence of voiding dysfunction in women increases with age (Haylen et al 2008b), and the process of ageing may decrease detrusor contractility and increase urethral rigidity.
Urodynamic studies have shown that both peak flow rate and detrusor pressure during voiding decrease with advancing age. Older women are also more likely to strain abdominally during voiding and to have higher residual urine volumes (Malone-Lee and Wahedna 1993).
It is not clear whether the menopause has an effect on voiding which is distinct from age. There are no studies showing that the menopause per se has a deleterious effect on voiding function. However, the distal urethra is oestrogen dependent and therefore susceptible to postmenopausal atrophic changes; the reduction in urethral functional length seen after the menopause is likely to be a manifestation of this process. There is evidence that these changes are more pronounced in a minority (18%) of postmenopausal women (Smith 1972). These women may have increased urethral rigidity and may be predisposed to the development of voiding dysfunction should they undergo pelvic or anti-incontinence surgery.
Postoperative voiding dysfunction
Pelvic surgery
With regards to specific procedures, clinical and urodynamic studies have found no evidence of increased voiding dysfunction in the short term after vaginal hysterectomy with anterior colporrhaphy performed at the same time (Stanton et al 1982), and after abdominal hysterectomy (Wake 1980). Also, no differences in bladder function were observed in a randomized study comparing total with subtotal hysterectomy (Thakar et al 2002). However, a history of previous hysterectomy has been associated with increased risk of voiding difficulty, possibly due to nerve dysfunction (Dietz et al 2002).
Extensive pelvic surgery might lead to denervation and prolonged or permanent voiding difficulty. Radical hysterectomy has been shown to lead to prolonged voiding difficulty in one-quarter of patients (Scotti et al 1986) due to neuropathic dysfunction.
Surgery for stress incontinence
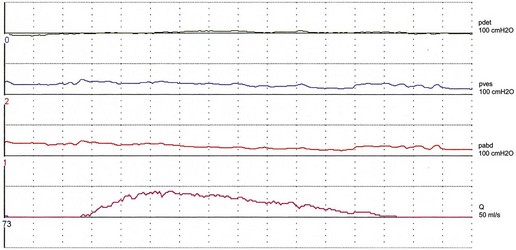
Women with stress incontinence may already have some impairment of voiding function, which may make them more vulnerable to the obstructive effects of surgery. This is suggested by differences in several urodynamic (voiding) variables noted in these women. For example, in contrast with normal women, women with stress incontinence are more likely to strain during voiding, and to initiate voiding with a Valsalva manoeuvre as opposed to pelvic relaxation. They also void with significantly lower detrusor pressures, and up to 15% have been shown to void without a contraction (Figure 54.3; Karram et al 1997). It is not clear whether this is due to the lower mean urethral pressure of women with urodynamic stress incontinence, or whether there is a real impairment of detrusor muscle function in these women.
Postoperative voiding disorders have been reported to occur after most operations for stress incontinence. Prolonged voiding dysfunction is uncommon after urethral injectables, although an incidence of 5% has been reported (Khullar et al 1997). The colposuspension operation leads to postoperative voiding dysfunction in a mean of 12.5% of patients (Jarvis 1994). Laparoscopic and open colposuspension have the same incidence of postoperative voiding difficulty (Carey et al 2006). Results of a randomized study comparing the tension-free vaginal tape (TVT) procedure with colposuspension suggest that the incidence of voiding difficulty at 6 months is similar after both operations (7%), although patients experience earlier voiding in the immediate postoperative period after the TVT procedure (Ward and Hilton 2002). After the TVT procedure, 11% of women have been reported to need a catheter for more than 24 h (Vervest et al 2007), with only 2% needing tape release for prolonged difficulty (Kuuva and Nilsson 2002, Karram et al 2003, Vervest et al 2007). The transobturator tape (TOT) procedure has been shown to have a lower incidence of postoperative voiding difficulty than the TVT, possibly as it is less obstructive (Latthe et al 2007).
Prolapse surgery
Short-term voiding difficulty is common after vaginal surgery for prolapse. In a large series of women undergoing such surgery, urinary retention (defined as a postvoid residual of ≥200 ml after removal of the catheter the day after the operation) occurred in 29% of women, with 9% experiencing retention for more than 3 days (Hakvoort et al 2009). In another study, 11% of women required catheterization at home after discharge from hospital (Vierhout 1998). However, no patients experienced long-term voiding difficulty (Vierhout 1998, Hakvoort et al 2009). Performing a levator muscle plication (as part of a posterior colporrhaphy) and a Kelly suburethral plication (as part of an anterior colporrhaphy) can increase the risk of postoperative retention (Hakvoort et al 2009).
A policy of early catheter removal, the day after vaginal surgery seems preferable to routine prolonged catheterization (4 days) as it is associated with a lower incidence of UTI and a shorter duration of hospital stay (Hakvoort et al 2004). However, early catheter removal increases the risk of recatheterization: 40% in the ‘early’ removal group vs 9% in the ‘late’ removal group (Hakvoort et al 2004). Prolonged catheterization may therefore be preferable in individual cases when there is an increased risk of postoperative voiding difficulty.
Postpartum voiding difficulty
Prolonged voiding difficulty in the postpartum period requiring self-catheterization is uncommon and has been reported to occur after less than 1% of deliveries (Glavind and Bjørk 2003). However, when using strict criteria [similar to those proposed recently by the International Urogynecological Association/International Continence Society (2010)], up to 43% of women have been shown to have some degree of voiding difficulty after delivery (Ramsay and Torbet 1993). Recognized risk factors for voiding difficulty in the immediate postpartum period are primiparity, instrumental delivery, epidural analgesia, prolonged labour, perineal trauma and poor bladder management resulting in overdistension. In addition to temporary factors (e.g. epidural, morphine, anaesthetic), other factors causing prolonged voiding dysfunction may be present (e.g. trauma to the bladder, pelvic floor muscles and nerves).
Neurological disease
Specific neurological conditions are discussed below.
Lumbar intervertebral disc prolapse
Lumbar disc prolapse is extremely common, with approximately 30% of adults without back pain having evidence of a protruded disc on magnetic resonance imaging (MRI) (Jarvik and Deyo 2007). In patients with symptomatic lumbar disc prolapse, urodynamic studies have shown voiding difficulty due to reduced detrusor contractility in approximately one-quarter of cases (Bartolin et al 1998). The most common sites of lumbar disc prolapse are L4–5 and L5–S1. Compression occurs more often in a posterolateral direction but may also be central. Patients may report a long history of low back pain, or voiding difficulty may be the first or only symptom. Compression of the sacral nerves can lead to cauda equina syndrome, characterized by voiding difficulty, saddle anaesthesia, bilateral sciatica and low back pain. Physical examination in these cases shows reduced sensation in the saddle and perianal area. If nerve compression is seen on MRI, urgent surgical treatment, usually laminectomy, is indicated. Despite this, voiding function often fails to improve after surgery (Bartolin et al 1999).
The lithotomy position commonly used to perform gynaecological procedures can potentially precipitate lumbar disc prolapse (Choudhari et al 2000). It has been suggested that flexion and hyperabduction of the hips may stretch nerve roots already compromised by a partial ‘occult’ disc prolapse (Choudhari et al 2000). When no other explanation can be found, this possibility should always be considered and investigated by MRI should a patient develop prolonged voiding difficulty after a gynaecological procedure.