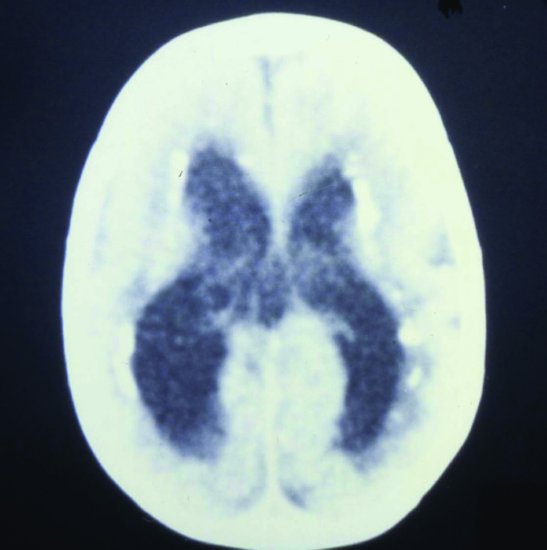
Figure 17.2 Congenital CMV. CT scan: periventricular calcification and ventricular dilatation (due to cerebral atrophy, not hydrocephalus).
Hepatitis and pneumonitis are rare in congenital infection and suggest CMV infection acquired post-natally (Section 17.1.4). They rarely occur concurrently.
17.1.3.2 Sensorineural hearing loss
Sensorineural hearing loss is commonest in symptomatic children, but asymptomatic infected infants can develop progressive sensorineural hearing loss. Severity is variable: unilateral or bilateral, mild to profound. Severity correlates with CMV DNA viral load.11 The mechanism of hearing loss is poorly understood.
A systematic review found approximately 14% of children with congenital CMV infection develop sensorineural hearing loss, which is bilateral moderate-to-profound in 3–5%.12 An estimated 15–20% of all cases of bilateral moderate-to-profound sensorineural hearing loss are caused by congenital CMV infection.12 In a large study from Brazil, a population with almost 100% CMV seroprevalence in pregnancy, the infection rate in newborns screened for congenital CMV infection was 10 per 1000 (1%) and 10% were symptomatic at birth.13 At 12 months, 9.8% had sensorineural hearing loss. Symptomatic infection at birth increased the risk of sensorineural hearing loss many fold (OR 38.1, 95% CI 1.6–916.7). In this population, only 4 of 10 infants with sensorineural hearing loss were born to mothers with primary CMV infection.13 Thus, congenital CMV infection is a major cause of sensorineural hearing loss in children worldwide, even in countries where most mothers are seropositive.
There is an ongoing debate whether or not to screen newborn infants and, if so, whether to screen them for hearing loss or congenital CMV infection.14 Onset of hearing loss is late in approximately 50% of congenital CMV and will not be detected by newborn hearing screening. Screening is unlikely to be worthwhile unless hearing loss is preventable (Section 17.1.5).
17.1.4 Clinical manifestations of post-natal cytomegalovirus infection
Post-natal CMV infection can result from maternal genital tract secretions at the time of birth, infected breast milk, blood products and respiratory exposure. It is usually asymptomatic although persistent infection with intermittent excretion is common. The level and route of virus exposure compared to intrauterine exposure may explain why post-natal CMV is less severe than congenital CMV infection. Infants born to CMV-seropositive mothers may also be relatively protected by transplacentally acquired CMV IgG. Manifestations include hepatitis, pneumonitis, sepsis, neutropoenia and thrombocytopaenia.15
17.1.4.1 Cytomegalovirus hepatitis
Hepatitis is unusual with congenital CMV infection but has been described, but is more characteristic of post-natally acquired CMV infection, particularly pre-term infants.16,17 In an observational study, signs and symptoms improved in seven infants treated with IV ganciclovir for 21 days but not in five untreated infants.16
17.1.4.2 Cytomegalovirus pneumonitis
CMV pneumonitis is characteristically afebrile and clinically and radiologically indistinguishable from Chlamydia pneumonitis (see Figure 8.2). Symptoms include increased oxygen requirement, tachypnoea and/or apnoea, cough, respiratory distress and increased respiratory secretions.10 There are no RCTs or even case series of ganciclovir in CMV pneumonitis.
17.1.4.3 Sepsis syndrome
In 1979, 16 (31%) of 51 infants <;1500 g screened for post-natal CMV excretion began excreting CMV in urine or nasopharynx after >28 days, and 14 of the 16 had a sepsis-like syndrome characterized by grey pallor, hepatosplenomegaly, respiratory deterioration and atypical lymphocytosis.18 The syndrome was thought to be caused by blood transfusion. In later studies, acquired CMV infection could be prevented by using CMV-seronegative blood donors19 or by filtering blood to remove white cells.20
17.1.4.4 Haematologic
Acquired CMV infection can result in persistent neutropoenia, thrombocytopaenia and lymphocytosis.21
17.1.5 Treatment of congenital cytomegalovirus infection
Studies of antiviral treatment for this condition are extremely difficult to perform. Treatment is prolonged, parenteral and toxic.
The recommended doses of antivirals based on pharmacokinetic data26 are
Ganciclovir 6 mg/kg/dose IV 12 hourly.
Valganciclovir 16 mg/kg/dose orally 12 hourly.
17.1.6 Prevention of congenital infection
A Cochrane systematic review27 of RCTs and quasi-RCTs found six studies of antenatal interventions for preventing the transmission of CMV from the mother to foetus during pregnancy including one using hyperimmune globulin,28 one using a candidate recombinant CMV envelope glycoprotein vaccine29 and one using parental education,30 but none met the study entry criteria.
17.1.7 Prevention of acquired cytomegalovirus infection
The rate of transfusion-acquired post-natal CMV infection can be reduced considerably by using CMV-seronegative blood donors19 or by filtering blood to remove white cells.20
17.1.8 Risk to staff
Pregnant nursing, medical and paramedical staff are often concerned about working with infants with congenital CMV infection but evidence shows health-care workers (HCW) are not at increased risk. In a longitudinal study the annual rate of seroconversion of seronegative medical students (0.6%), house staff (2.7%) and nurses (3.3%) was not significantly higher than young women in the community (2.5% during pregnancy and 5.5% between pregnancies).31 In a review of similar serologic studies the annual seroconversion rate ranged from 1% to 7% of pregnant women (summary annual rate 2.3%) and was also 2.3% in HCWs, including those caring for infants and children.32 Most parents exposed to a CMV-shedding child do not become infected. However, seroconversion rates were higher in day-care providers (range 0–12.5%, summary annual rate 8.5%),32 perhaps because exposure is greater and/or hand hygiene is poorer in day care.
We recommend not evaluating CMV serostatus routinely in pregnant HCWs, because their risk is no higher than the general population. Congenital CMV can follow primary or post-primary maternal infection, so serologic interpretation is complex. However, serology for CMV IgM may be indicated if a pregnant HCW develops a glandular fever-like illness.
17.2 Herpes simplex virus
Herpes (Greek) means both ‘creeping like a snake’ and ‘latent’. Spreading skin lesions may appear to creep. The ancient Greeks knew of herpes infections and Herodotus used the term herpes febrilis to describe the association between oral herpes and fever.
Neonatal herpes simplex virus (HSV) infection was first described in the 1930s. HSV can cause blistering skin lesions, conjunctivitis, mouth blisters and ulceration, isolated pneumonitis or a sepsis-like syndrome with thrombocytopaenia. Suspicion needs to be high because, unless treated early, localized infections usually disseminate and mortality is high.
17.2.1 Virology
HSV, like all herpesviruses, can cause latent (hidden) infection and reactivate with intermittent viral shedding. It was recognized in the mid-1960s that there were two antigenic types of HSV (HSV-1 and HSV-2) and that infection with one provided at best partial protection against infection with the other type.33
HSV is neurotropic: the site of latency is sensory neurones. Primary infection is followed by productive replication in mucosal epithelial cells. HSV enters the sensory neurones through nerve terminals, is transported to neuronal cell bodies and establishes latency. HSV can reactivate intermittently resulting in new virus progeny that are transported axonally back to the periphery. The ability to establish lifelong latency and reactivate periodically to facilitate dissemination is central to the virus’ survival strategy. The molecular mechanisms are only partially understood.34
17.2.2 Epidemiology
HSV needs to be in contact with mucosa or broken skin to initiate infection, generally requiring person-to-person contact. HSV spreads readily among infants and toddlers in day care through infected secretions or respiratory droplets.
HSV can cause classic transplacental congenital HSV infection but neonatal HSV infection is far more commonly acquired perinatally. True congenital HSV infection due to transplacental infection is responsible for around 3–5% of neonatal HSV infections; the remaining 95–97% are post-natally acquired.35
Neonatal HSV infection can occur following maternal primary infection or reactivation. In a study from Sweden and the United States, HSV-1 was far more readily transmissible to the neonate than HSV-2 during reactivation (adjusted pooled OR 19.2; 95% CI 5.8–63.6), and the authors expressed concern in view of the rising frequency of genital HSV-1 infection.33
Maternal IgG antibody is relatively but not absolutely protective against neonatal HSV infection. In a prospective study, HSV was isolated from 56 (0.35%) of 15 923 genital viral cultures performed on women in early labour with no symptoms or signs of genital HSV infection.36 There was serologic evidence of subclinical primary genital HSV infection in 18 (35%) of the 56, while 34 (65%) had HSV reactivation. Six of 18 infants (33%) of women with primary genital HSV developed neonatal HSV compared with 1 of 34 infants (3%) of women with HSV reactivation (p <; 0.01). The risk of neonatal HSV was increased in association with proven viral shedding from the cervix and with use of foetal scalp electrodes.36
In another large prospective US study, HSV was isolated from 202 women (5 per 1000) at the time of labour and 10 (5%) of them had neonates with HSV infection.37 Risk factors for neonatal HSV included first-episode infection (OR 33.1; 95% CI 6.5–168), HSV isolation from the cervix (OR 32.6; 95% CI 4.1–260), HSV-1 versus HSV-2 isolation at the time of labour (OR 16.5; 95% CI 4.1–65), invasive monitoring (OR, 6.8; 95% CI, 1.4–32), delivery before 38 weeks (OR 4.4; 95% CI 1.2–16) and maternal age <;21 years (OR 4.1; 95% CI 1.1–15). Neonatal HSV infection rates per 100 000 live births were 54 (95% CI 19.8–118) among HSV-seronegative women, 26 (95% CI 9.3–56) among women who were HSV-1 seropositive only and 22 (95% CI 4.4–64) among all HSV-2-seropositive women. The HSV transmission rate was 1 of 85 (1.2%) delivered by caesarean section and 9 of 117 (7.7%) delivered vaginally (OR 0.14; 95% CI 0.02–1.08).37
The incidence of neonatal HSV infection was 9.6 per 100 000 live births in 2006 in the United States38 and 5.9 per 100 000 in Canada from 2000 to 2003.39 The incidence of HSV-1 infection is rising in many countries in adults, and so is the proportion of neonatal HSV infections due to HSV-1.40
17.2.3 Clinical presentation
17.2.3.1 Congenital herpes simplex virus infection
The classic triad of congenital HSV infection presenting at birth is involvement of the skin (vesicular or bullous skin lesions), eye (chorioretinitis and/or keratoconjunctivitis) and CNS (microcephaly). A literature review yielded only 64 published cases of intrauterine HSV infection, of which less than one-third fit the typical triad.35 Almost half (44%) of the infants with skin manifestations had lesions other than vesicles or bullae. Diagnosis was delayed >3 days after birth in 15 infants (23%) with the median age 10 days. HSV was often not considered in the differential diagnosis of infants with birth skin lesions that were not vesicles or bullae. New vesicles appeared in a number of infants.
A rare but important manifestation of congenital and neonatal HSV infection is with hepatomegaly and cytopaenia of two or more cell lines due to hemophagocytic lymphohistiocytosis or HLH (see Section 18.6).41 If HSV is recognized early as the cause of HLH, acyclovir treatment is often curative.
17.2.3.2 Neonatal herpes simplex virus infection
Neonatal HSV infection is usually characterized as localized skin, eye or mouth (SEM) disease; encephalitis; or disseminated infection. HSV can also present in week 1 (‘5-day pneumonitis’) or 2 as isolated respiratory distress. Encephalitis and disseminated infection have a very poor prognosis. Localized disease including pneumonitis progresses to disseminated disease if not recognized and treated early.
17.2.3.2.1 Skin, eye and mouth infections
The hallmark of HSV skin lesions is the tendency to form individual punched-out vesicles about 0.5 cm in diameter which often rupture and coalesce (see Figures 13.1d, 13.1e and 17.3). Lesions can be anywhere on the infant’s skin (Figure 17.3). The scalp is a common site (see Figures 13.1d and 13.1e). It is important to have a high index of suspicion and a low threshold for investigation of neonatal skin lesions. Lesions around the eye are suspicious, particularly if there is associated conjunctivitis (see Figures 12.3a, 12.3b and 17.4). Dendritic ulcers are a late manifestation of HSV eye infection (see Figure 12.4). Oral ulceration due to HSV in neonates is much less commonly reported than skin and eye lesions, because it is truly rare, atypical or oral examination is performed badly. Rapid diagnosis is essential (Section 17.2.4).
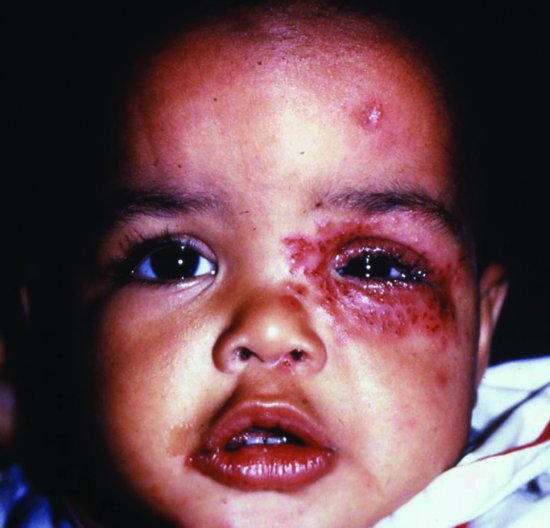
Figure 17.4 HSV infection of eye and skin. Left eyelid swollen and inflamed. Periorbital herpetic skin lesions have coalesced. Skin lesions also present on forehead and lips.
Finding localized disease does not guarantee a good outcome. In a case series around 10% of infants with SEM disease have had long-term neurologic impairment, sometimes severe. The reason is uncertain: undiagnosed acute CNS infection and progressive CNS disease due to chronic or recurrent HSV infection are possible mechanisms.
17.2.3.2.2 Herpes simplex virus pneumonitis
Isolated HSV pneumonitis presents with respiratory distress 3–14 days after birth and is often misdiagnosed as bacterial pneumonia, even when this is clinically unlikely, or as chlamydial or viral pneumonia. The classic chest radiograph shows hilar and central interstitial infiltrate (see Figure 8.3), but HSV pneumonitis is not distinguishable radiologically from other pneumonitides.42 Rapid viral diagnosis should be performed on respiratory secretions (nasopharyngeal or endotracheal secretions) using immunofluorescence, ELISA or PCR. If the test is positive for HSV, or rapid tests are not available or even if there is a negative test but high clinical suspicion, empiric acyclovir therapy should be started pending further investigation.
17.2.3.2.3 Herpes simplex virus encephalitis
Encephalitis is the sole manifestation of about a third of neonatal HSV infections or can occur with disseminated infection. Infants with neonatal encephalitis usually present in the second week of life, occasionally in the first week or >14 days with seizures, fever, lethargy, poor feeding, irritability, jitteriness and rigidity. The presentation of HSV-2 encephalitis is more fulminant than HSV-1 and the outcome poorer.43–45
LP usually shows a mononuclear cell pleocytosis although acellular CSF has rarely been reported from infants with HSV on brain biopsy (in the days before brain biopsy was replaced by PCR). HSV encephalitis is often haemorrhagic, resulting in micro- or macroscopic blood in the CSF. CSF glucose may be low and protein may be normal initially but rise with illness progression. Although false positives do rarely occur, a positive CSF PCR is an absolute indication for acyclovir therapy. CSF PCR is positive in at least 70% of infants with HSV encephalitis, but may be negative early in the course; as a negative PCR does not exclude HSV encephalitis acyclovir treatment may be necessary until a repeat LP can be performed.45,46
Brain imaging may show temporal, parietal, frontal or sub-cortical parenchymal damage. Although temporal changes are common in HSV encephalitis, there are no specific radiologic changes that distinguish HSV reliably from other causes of encephalitis.
17.2.3.2.4 Disseminated herpes simplex virus infection
The clinical features of disseminated HSV infection overlap with severe bacterial sepsis. Infants with disseminated HSV infection often have hepatitis and features of disseminated intravascular coagulopathy such as thrombocytopaenia and a bleeding tendency. Other clinical features such as jaundice, irritability, seizures and shock may not be specific enough to alert the clinician to the diagnosis. Rash is rarely present initially although it may develop. HSV may be detected by PCR or cultured from peripheral blood: a high index of suspicion is the clue to diagnosis.
17.2.3.2.5 Hemophagocytic lymphohistiocytosis
Neonatal HSV infection can occasionally cause hemo-phagocytic lymphohistiocytosis (HLH) presenting clinically with hepatomegaly and/or splenomegaly and possibly fever, lymphadenopathy, respiratory failure or seizures.41 Affected infants have cytopaenia of two or more cell lines and greatly elevated serum ferritin levels. Hemophagocytosis is often difficult to prove, but biopsies of bone marrow, lymph nodes (see Figure 18.10) or spleen may be characteristic. If HSV infection is the cause, diagnosis is critical to initiate acyclovir treatment (see Section 18.6).
17.2.4 Diagnosis
Rapid virologic techniques (e.g. nucleic acid amplification by PCR; antigen detection by ELISA; immunofluorescence) have revolutionized the diagnosis of neonatal HSV infection. HSV culture is rarely performed in many centres, because although sensitive it is not timely. PCR can be performed on most body fluids including blood and CSF.
17.2.5 Treatment
A Cochrane systematic review of antivirals for neonatal HSV infection47 found two RCTs (273 infants). One study compared vidarabine with placebo in 63 infants.48 There was no significant reduction in mortality overall, but mortality was significantly reduced in infants with either CNS or disseminated disease. There was no difference in the rate of neurologic abnormalities in survivors at 1 year when analysed as an entire group or by disease category.48
An RCT comparing acyclovir and vidarabine in 210 infected infants found no difference in mortality, disease progression, incidence of neurologic abnormality at 1 year or incidence of drug-induced renal or bone marrow toxicity. In infants with SEM disease, there was no significant difference in neurologic outcome.49 Despite the similar outcomes, acyclovir is the only agent used nowadays because of the prohibitive volume of administration of vidarabine.
Current guidelines, based on pharmacokinetic data, recommend treatment with intravenous acyclovir (20 mg/kg/dose 8 hourly). Localized disease is treated for 14 days, provided that investigations include CSF examination to exclude CNS involvement. Disseminated disease and encephalitis should be treated for 21 days. It is recommended to monitor the neutrophil count and, if the absolute neutrophil count falls below 500/mm3, decreasing the acyclovir dose or giving granulocyte colony stimulating factor (G-CSF) should be considered.
17.2.6 Prognosis
Despite concern that antivirals might increase survival at the cost of increased neurologic morbidity, one RCT48 and observational studies have suggested this is not the case.51
Prior to antiviral therapy, the 12-month mortality of disseminated HSV disease was 85% and of HSV encephalitis was 50%. Since high-dose acyclovir has been used, the 12-month mortality for disseminated neonatal HSV has fallen to 29% and for HSV encephalitis to 4%.51,52 Morbidity has improved compared to historic data, but remains high. Most survivors of disseminated HSV disease (83%) but only 31% of survivors of HSV encephalitis are assessed as having normal neurodevelopment.53 The morbidity of SEM disease has improved and <;2% of survivors have impaired neurodevelopment.53
17.2.7 Prevention of neonatal herpes simplex virus infection
Unfortunately there is no effective vaccine commercially available yet. A trial of a herpes simplex virus type 2 (HSV-2) subunit vaccine containing glycoprotein D in women aged 18–30 years seronegative for HSV-1 and HSV-2 surprisingly showed that although the vaccine did not protect against HSV-2, HSV-1 genital disease was reduced by 58% (95% CI 12–80) and HSV-1 infection (with or without disease) by 35% (95% CI 13–52).54
A Cochrane systematic review of antenatal antiviral prophylaxis for recurrent genital herpes found seven eligible RCTs (1249 participants) which compared acyclovir to placebo or no treatment (five trials) and valaciclovir to placebo (two trials).55 There were no cases of symptomatic neonatal herpes in either treatment or placebo groups. Women who received antiviral prophylaxis were less likely to have a recurrence of genital herpes at delivery and less likely to have a caesarean delivery for genital herpes.55
If an asymptomatic mother is shedding HSV, transmission can be reduced several fold by caesarean delivery (Section 17.2.2). If the mother has symptomatic genital HSV infection, caesarean section is certainly recommended for primary infection. However, even if a history of prior genital herpes proves the mother’s symptoms are due to reactivation, caesarean is recommended because symptomatic infection is associated with a high viral load which may swamp any passively acquired maternal antibody.37 Neonatal transmission can be reduced by limiting the use of invasive monitors among women shedding HSV at the time of labour.37
17.3 Varicella zoster virus
Varicella zoster virus (VZV) shares the ability of all herpesviruses to cause acute infection but also lie dormant in peripheral nerves and reactivate intermittently. Acute infection is called varicella or more colloquially chickenpox; reactivation disease is called zoster or herpes zoster. Varicella and zoster are caused by the same virus, hence varicella zoster virus. VZV is important in perinatology for three reasons:
- Maternal VZV primary infection in pregnancy can be life-threatening.
- Maternal VZV primary infection can cause congenital varicella syndrome.
- Neonatal VZV infection can be life-threatening.
Varicella zoster immunoglobulin (VZIG or ZIG) is an immunoglobulin preparation derived from blood donors with high VZV antibody titres. In 1969 VZIG was shown to protect healthy children against chickenpox.56 It has been shown since then that VZIG does not always prevent chickenpox but reduces its severity. Passive protection is particularly important for unimmunized hosts with impaired T-cell function at risk of severe VZV infection. Both pregnant women and their newborns have relatively poor T-cell function.
17.3.1 Maternal primary varicella zoster virus infection in pregnancy
Primary VZV infection in pregnancy is relatively rare. The incidence of chickenpox in pregnant women in the United Kingdom has been estimated to be at least 1 per 2000 pregnancies.57 The true incidence depends on local epidemiology, including whether or not countries have routine infant VZV immunization programmes, and if not whether most of the population are infected in childhood.58–60 Primary VZV in adolescence or adulthood can be complicated by life-threatening pneumonitis and the immunosuppressive nature of pregnancy may predispose to severe primary infection. In a case series of adults without severe immunodeficiency managed in a high dependency unit for chickenpox pneumonitis, 30% were pregnant women.61 However, there are no definitive studies showing chickenpox is more severe in pregnancy than in non-pregnant adults.
An evidence-based systematic review of prevention and treatment of VZV in pregnancy from Canada62 recommended varicella immunization for all non-immune women as part of pre-pregnancy and postpartum care. VZV is a live attenuated vaccine and so, although vaccination within 2–3 days of exposure is protective,63 immunization in pregnancy is not recommended. However, the vaccine is probably not teratogenic and termination of pregnancy should not be advised because of inadvertent vaccination during pregnancy.
A pregnant woman with unknown VZV serostatus exposed to VZV should have serologic testing. If the mother is seronegative or the serum result is unavailable within 96 hours from exposure, varicella zoster immunoglobulin (ZIG or VZIG) should be administered,62 because VZIG reduces the risk both of the pregnant woman developing pneumonitis62 and of congenital varicella (Section 17.3.2).64 Prophylactic acyclovir is not recommended for maternal VZV exposure due to safety concerns, although it should be effective if VZIG is unavailable. Normal human immunoglobulin and intravenous immunoglobulin are other alternatives to VZIG.
Women who develop varicella infection in pregnancy should be counselled of the potential adverse maternal and foetal sequelae, the risk of transmission to the foetus and the options available for prenatal diagnosis. It is recommended that all women who develop varicella in pregnancy have serial ultrasounds to screen for congenital varicella infection.59 Women with significant varicella infection in pregnancy should be treated with acyclovir; pneumonitis is usually an indication for hospital admission and IV acyclovir.62
17.3.2 Congenital varicella syndrome
Congenital varicella syndrome is characterized by cicatricial (scarring) skin lesions and/or hypoplasia of a limb and severe CNS (microcephaly, cortical atrophy, seizures, developmental delay) and ocular abnormalities (chorioretinitis, microphthalmia).65,66 The cicatricial limb lesions may represent scarring from intrauterine zoster (Figure 17.5).
Figure 17.5 Cicatricial skin lesion of congenital varicella syndrome (possibly due to in utero zoster).
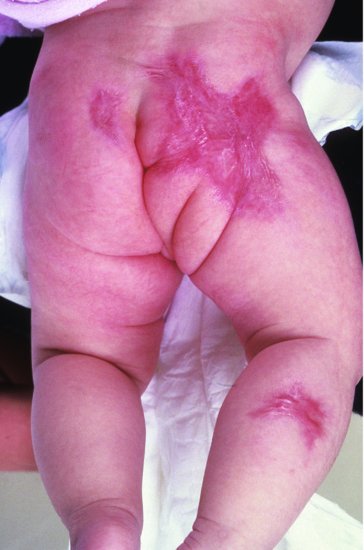
Diagnosis of congenital varicella syndrome is primarily clinical. VZV-specific IgM antibody was detected at birth in 4 (25%) of 16 infants with clinical congenital varicella syndrome and persistent specific IgG antibody in 5 of 7 infants tested.11 The viral load in congenitally infected infants is much lower than in congenital rubella or CMV and PCR for VZV is diagnostically unreliable.
The strongest evidence on the risk of congenital varicella syndrome following maternal chickenpox or zoster comes from a large prospective study in Germany and the United Kingdom.67 Nine babies with congenital varicella syndrome were all exposed to maternal varicella in the first 20 weeks of gestation. The highest risk was observed between 13 and 20 weeks of gestation, with 7 of 351 affected pregnancies resulting in congenital varicella (risk 2%, 95% CI 0.8–4.1). Two of 472 infections <;13 weeks resulted in congenital varicella (risk 0.4%, 95% CI 0.05–1.5). A smaller US study (106 women with clinical varicella) reported the risk of varicella embryopathy following maternal varicella <;20 weeks was 1.2% (95% CI 0–2.4).68
None of 366 infants born to women with herpes zoster in pregnancy developed congenital varicella (risk 0; 95% CI 0–1).67 Congenital varicella syndrome has never been described following maternal zoster, so mothers can be counselled that the risk from maternal zoster is almost negligible.
A non-Cochrane systematic review64 of the efficacy of maternal VZIG in preventing congenital varicella syndrome found three studies.65,69,70 Congenital varicella syndrome developed in 14 (2.8%) of 498 infants whose mothers were not given VZIG but in no foetus or infant born to 142 mothers who received VZIG (p <; 0.01 by Fisher exact test).64
This supports the recommendation to give VZIG to non-immune pregnant women exposed to VZV in the first 20 weeks of gestation to protect the foetus (Section 17.3.1) as well as the mother.
17.3.3 Neonatal varicella
Some infants whose mothers have perinatal chickenpox develop pneumonitis with a high mortality (Figures 17.6a and 17.7) while others are only mildly infected (Figure 17.6b
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree