New cases of viral hepatitis are prevented
Persons who are already infected are tested
Informed about their infection
Provided with counseling, care, and treatment
Educate providers and communities to reduce health disparities
Improving testing and link to care to prevent HBV-related liver disease
Eliminate vertical transmission
The Pregnant Patient with Hepatitis B
Screening and Diagnosis
The prevalence of HBsAg positivity in pregnant mothers mirrors the general population (Table 9.2) [26] and varies by race: 6 % Asian, 1 % Black, 0.6 % Caucasian, and 0.14 % Hispanic.
Table 9.2
Prevalence of HBsAg positive among pregnant women
Mirrors the prevalence in the adult community |
In the United States, varies by race |
• 6 % Asian |
• 1 % Black |
• 0.6 % White |
• 0.14 % Hispanic |
In American-born patients, the most likely source of infection is drug use or sexual transmission, whereas patients born in endemic areas have most likely acquired the infection at birth. Foreign-born individuals represent the majority of HBsAg-positive mothers. In the United States, there is universal screening for HBV infection in all pregnant women with an estimated 96 % of all pregnant women tested. Mothers who have not had antenatal testing are tested at delivery by the birthing hospital [27].
Counseling
For many patients, prenatal screening coincides with the time of initial diagnosis. The role of the physician in this instance is of increased importance given the emotional implications of the diagnosis (Table 9.3). Moreover, given the multicultural background of the patients, the counseling must be provided in a context that is sensitive to this diverse and vulnerable population.
Table 9.3
The HBsAg-positive patient: initial encounter key points
Education • Natural history of chronic hepatitis B: discuss long-term complications and available therapies • Discuss pregnancy outcomes • Vertical transmission prevention: discuss immunoprophylaxis and immunoprophylaxis failure • Household contacts: encourage vaccinations |
Action • Initial evaluation of chronic hepatitis B and referral to the specialist for consultation • Report the patient to the Department of Health and to the DOH Perinatal Hepatitis Prevention Program |
Reassurance regarding the likelihood of good outcomes in pregnancy and education regarding transmission inside the household and to the unborn child must be provided. Moreover, pregnancy is a unique and in many cases the only opportunity for these patients to be educated about hepatitis B, its long-term effects, and the available therapies.
Pregnancy Outcomes
Effect of HBV Infection on Pregnancy
It is thought that hepatitis B virus infection does not adversely affect the course of pregnancy, nor does pregnancy adversely affect the natural history of the disease. Indeed large retrospective cohorts have shown no difference in maternal or fetal outcomes compared to healthy non-HBV-infected controls. However, acute HBV infection is associated with high vertical transmission rates when occurring in the third trimester and increased incidence of low birth weight and prematurity [28]. Moreover recent studies suggested an increased risk of preterm birth, antepartum hemorrhage, and gestational diabetes in mothers chronically infected with hepatitis B [29, 30]. However, it is possible that the underlying advanced liver disease itself contributed to the high complication rate rather than the HBV infection itself.
Effect of Pregnancy on Hepatitis B Virus-Related Liver Disease
HBV-related liver disease typically does not worsen during pregnancy. However, a small percentage of patients may develop cholestasis, chronic hepatitis B flare, or liver failure [28, 31]. During normal pregnancy, increased HBV viral load has been observed, leading to minor fluctuations in liver function tests and thought to be secondary to high levels of adrenal corticosteroids and estrogen hormones [32].
In one study, HBV virus levels remained stable, but ALT levels increased particularly in late pregnancy and postpartum periods. Further evidence supports the postpartum period as the most vulnerable time for HBV exacerbations. In a Japanese study, of 269 pregnant patients with chronic HBV infection, alteration of liver function was found in 43 % during the first month postpartum with some exacerbations leading to HBV seroconversion. Given the uncertainty of the natural history of HBV infection during and after pregnancy, patients should be monitored closely during pregnancy and postpartum.
Evaluation the Underlying Liver Disease
Although pregnancy is not contraindicated in HBV-infected individuals or for patients with severe liver disease, a comprehensive assessment of the underlying chronic hepatitis B is advised for all HBsAg-positive patients. This will serve two purposes: first to detect cirrhosis and potential liver insufficiency that will complicate the pregnancy, second to inform the patient about her disease and the available therapy, and third to set up appropriate follow-up care.
The recommendation endorsed by The American College of Obstetrics and Gynecology is that all HBsAg-positive mothers should be referred during pregnancy to a physician familiar with evaluation and treatment of viral hepatitis. Unfortunately, in a recent survey, only a third of patients were seen for consultation [33].
HBsAg positivity is associated with advanced liver disease in 30 % of all patients. Assessing the extent of the liver disease is particularly challenging because it usually requires liver biopsy and histological analysis. Liver biopsy may be performed in this population, because the risks are same as in a nonpregnant patient; however, invasive testing is usually avoided during pregnancy unless absolutely necessary. The use of ultrasound and other newer noninvasive predictor of advanced liver damage such as MRI, FibroScan, and FibroSure have not been validated in pregnancy. The diagnosis thus relies on clinical and laboratory data (Table 9.4).
Table 9.4
Initial evaluation of the HBsAg-positive patient
History | Risk factors Previous history of HBsAg+ Treatment history | |
Physical exam | Presence of signs and symptoms of chronic liver disease | |
Laboratory tests | Viral studies | • HBc Ab IGM and total, HBeAg, HBeAb, HBV, DNA • HDV, HIV, HCV serology |
Liver function | • CBC with platlets, PT/INR, ALT, AST, total bilirubin | |
Imaging | Liver damage | Right upper quadrant U/S |
The immunoglobulin M class of hepatitis B core antibody (HBcAb) is the first to appear during acute infection, and its presence is used as the serologic marker of acute HBV infection. The presence of HBeAg positivity is associated with high infectivity and presence of high number of viral particles in the circulating blood (viral load). The presence of HBeAb positivity is associated with low infectivity, low replicative state, and low or undetectable viral load. Viral load is considered the most reliable indication of viral replication. HBV serological markers and their significance are reviewed in Table 9.5.
Table 9.5
Serological markers of HBV infection
HBsAg | Component of viral envelope | Indicator of disease |
Anti-HBs | Antibody response to HBsAg | Indicates recovery and acquired immunity |
HBeAg | Antigen that correlates with replication and infectivity | High level of infectivity and replication |
Anti-HBe | Antibody response to HBeAg | Decreasing level of replication Remission/resolution High replication in mutant strains |
Anti-HBc IgM | Nonprotective antibody to the HBcAg Early marker of infection | Acute HBV infection |
Anti-HBc IgG | As above | Previous or current HBV infection |
HBV DNA | Replicative genetic material of HBV infectious agent | Viral replication and continues infection |
HBV viral load range is wide going from undetectable in inactive carriers to billions of viral copies in highly infective individuals. The definition of “high viral load” is not standardized. In general, a viral load exceeding 10 to the sixth copies/mL or >200,000 IU/ml is considered high, but several scientific publications and guideline use the terminology of “high viral load” for values from 10 to the fifth to 10 to the eighth.
HBsAg-positive patients with normal LFT’s, platelets, and liver synthetic functions and low or undetectable viral load are inactive carriers. It is important to remember that they still need to be followed yearly since they are at risk for reactivation and hepatocellular carcinoma.
Vertical Transmission
Overall, the virus infects 75 % of all babies born from HBsAg-positive mothers in the absence of immunoprophylaxis. If the mother has a high viral load, as is the case in hepatitis B eAg-positive patients, this number is more than 90 %. However, newborns from mothers who are healthy carriers with minimal viral replication and hepatitis BeAb positivity are infected in less than 10 % of cases [34]. In the United States, the vertical transmission of hepatitis B has been reduced dramatically by the implementation of a nationally funded Perinatal Hepatitis Prevention Programs. Table 9.6 summarizes the steps.
Table 9.6
Immunoprophylaxis: current recommendations
Mandatory testing for HBsAg in all pregnant patients (implementation in 19 states. Proof of test required in some states to obtain birth certificate) |
During pregnancy, vaccinate uninfected mother at risk. Retest them during the third trimester |
Postexposure immunoprophylaxis of infants born to HBsAg+ mothers with HBIg in 12 h from birth and HBV vaccine at birth, completion of subsequent doses (at least 2) within 6 months. Second dose in 10 weeks |
Infants retested for HBsAg and HBsAb at 9 months to exclude vertical transmission and ensure adequate antibody titer |
Perinatal Hepatitis Prevention Programs with “Case Managers” performing education and follow up |
Currently, available vaccines are safe in pregnancy, and mothers who are at risk for acquiring infection during pregnancy (injection drug users, patients with multiple sexual partners or who are infected with other sexually transmitted diseases] should be vaccinated and retested for HBsAg again in the third trimester close to the time of delivery [35]. Passive immunoprophylaxis with HBIG is also safe in pregnancy and should be given to women with documented exposure. Management of babies born from HBsAg + mothers (including preterm infants) is outlined above. This schedule has achieved nearly a 97 % success rate in preventing overall vertical transmission [36].
Immunization Failure
Despite HBIG administration and vaccination, the rate of HBV infection in the newborn may still reach 8.5 % in mothers with high viral load [37]. CDC estimated that 1,000 newborns are infected vertically each year in the United States. In a recent study, all cases of immunoprophylaxis failure occurred in mothers with high viral load (defined as >106 copies/mL) [38]. The reason for immunoprophylaxis failure is that multifactorial prophylaxis regimens have been implemented in only 62 % [39] of cases. Vertical transmission of HBV occurs during delivery from blood-to-blood contact and occasionally in uteri by transplacental translocation of the virus [40]. Amniocentesis risk is controversial and not well established, but the test should be performed only if absolutely necessary [41].
Delivery and Breastfeeding
Elective cesarean session has been found in some studies to be protective from immunization failure, and it is advocated for mothers with high viral load and risk of vertical transmission [42]. However, this recommendation is not generally accepted, and conflicting data still exist in the literature. Specific guidelines do not exist regarding the mode of delivery [43, 44].
Breastfeeding has been a major concern for source of transmission; however, several studies have refuted this and shown that breastfeeding carries no additional risk to transmission of the virus in the setting of prompt newborn immunoprophylaxis and vaccination [45].
Antiviral Therapy in Pregnancy
Antiviral agents are used in pregnancy both to treat the expectant mother and to reduce vertical transmission.
Antiviral for Maternal Benefit
Decisions regarding therapy initiation, continuation, and termination should be the same for pregnant patients and general population. Antiviral therapy in pregnancy, however, is in general postponed until after birth unless necessary for treatment of acute flares or advanced liver disease. Successful treatment of liver failure in pregnancy has been described with antiviral therapy.
The discontinuation of treatment will result in rebound increasing viremia and subsequent disease flares. Continuation of the therapy in pregnancy is prudent especially in view of their relatively safe profile and the risk of fulminant hepatic failure associated with disease flares in pregnancy and postpartum [24] (Table 9.7).
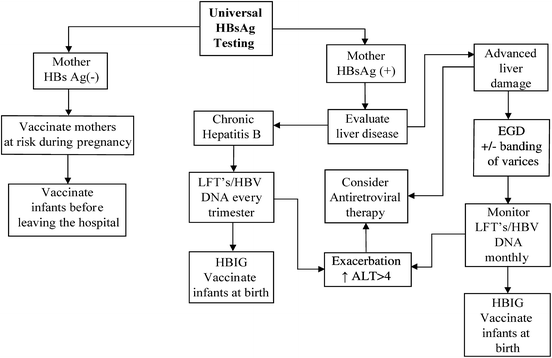
Table 9.7
Algorithm for treatment of hepatitis B in pregnancy

Antiviral Therapy to Reduce Vertical Transmission
Immunization failure has been prevented by short courses of antiviral therapy during the second and third trimester of gestation in a few studies [46, 47]. In this patient population of high viral load mothers with otherwise limited underlying liver damage, therapy was initiated solely to prevent immunization failure.
Current use of antiviral to decrease vertical transmission is endorsed by many experts and was incorporated in the 2012 EASL Guidelines. This strategy is not uniformly accepted. Substantial debate still exists regarding the patient population to treat, choice of drug, length of treatment, and appropriate follow-up [48].
Two meta-analyses concluded that lamivudine treatment from 24 to 32 weeks of gestation until 1 month postpartum in those mothers who had high viral loads (>106) was not only safe but more effective than HBIG and vaccination of the newborn alone [43, 49]. Most recently, a meta-analysis included four randomized controlled trials with a total of 306 mothers showing that telbivudine given late in pregnancy was safe as well as effective in reducing vertical transmission [50].
Safety of Antiviral Therapy
The safety data for antiviral therapy is derived from the antiretroviral pregnancy registry (APR) and the Development of Antiretroviral Therapy Study (DART). No significant differences in rates of adverse outcomes have been reported for HB antiviral drugs initiated throughout the three trimesters of pregnancy [51]. These rates were comparable to the defect rates in the general population (reported by the CDC).
Lamivudine and tenofovir have the largest data set because they have been used in HIV-infected patients. Tenofovir and entecavir are the most potent drugs with less likelihood of developing resistance and the ones recommended to treat nonpregnant patients. Tenofovir has been associated with skeletal abnormalities [52], and further studies assessing newborn bone density are planned. Limited data are available for entecavir and adefovir use in pregnancy Table 9.8.
Table 9.8
Birth defects associated with antiviral exposure (APR report, July 2013)
Antiviral agent | Pregnancy category | Live birth earliest exposure first trimester | Prevalence birth defects |
|---|---|---|---|
Telbivudine | B
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|