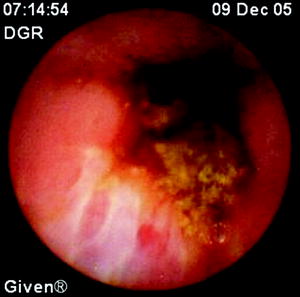
Fig. 22.1
Normal mucosal findings (a) in the mid small bowel as seen by wireless capsule endoscopy in a child suspected of Crohn disease. The astonishing resolution of the capsule’s lens (0.1 mm) affords visualization of the normal villi and mucosal blood vessels. In contrast, subtle inflammatory changes of the small bowel mucosa that were not visualized radiologically can readily be seen focally by capsule endoscopy. There include areas of mucosal nodularity with focal villous atrophy as well as “white tipped villi,” signifying inflammatory edema (b), as well as superficial linear ulcerations (c). Whereas these lesions detected by capsule endoscopy are typical of Crohn disease, they may be caused by other etiologies, including the use of medications such as nonsteroidal anti-inflammatory drugs
Potential Uses of Capsule Endoscopy for Inflammatory Bowel Disease
The diagnosis of IBD entails the documentation of the extent as well as severity of the inflammation affecting segments of the gastrointestinal tract, as well as the exclusion of other etiologies. There is no single test that is pathognomonic for ulcerative colitis (UC) or Crohn disease (CD). Bowel imaging techniques, whether endoscopic or radiological, are employed to support the diagnosis. The combination of ileocolonoscopy and EGD with multiple biopsies can usually differentiate between UC and CD, based on the distribution and pattern of mucosal inflammation [1, 3]. Endoscopic procedures provide invaluable information regarding the anatomic extent and severity of the mucosal inflammation. However, the vast majority of the small bowel is inaccessible to standard endoscopy or even enteroscopy. Over the past few years, several studies have established the utility of CE to evaluate the small bowel in patients with IBD [11]. The potential uses of CE in established or known IBD are summarized in Table 22.1, and discussed below.
Table 22.1
Potential indications for capsule endoscopy in IBD
Diagnosis of suspected small bowel Crohn disease |
Determination of the extent and severity of small bowel disease in established Crohn disease* |
Evaluation of the presence of small bowel lesions in patients with colonic inflammatory bowel disease (ulcerative or indeterminate colitis, IBD-U) |
Evaluation of mucosal healing of small bowel Crohn disease after treatment |
Assessment of postoperative recurrence of small bowel Crohn disease |
Incomplete colonoscopy |
Diagnostic Utility in Suspected Crohn Disease
Contrast small bowel radiography (SBR) and endoscopy (EGD and ileocolonoscopy) have long been the standard methods for evaluating known or suspected small bowel CD [1, 3]. However, SBR has relatively low sensitivity for early and superficial lesions of CD in the small bowel [9, 10]. Ileoscopy, when achieved, generally only affords examination of the terminal ileum. Push enteroscopy can be employed to examine the proximal regions of the small bowel that cannot be examined by EGD. However, it too has a rather limited range. Consensus statements suggest that CE is a far more promising tool for the evaluation of the small bowel in IBD [11, 12]. In cases where prior traditional investigations including EGD, ileocolonoscopy and SBR were generally negative or nondiagnostic, CE is vastly superior to establish, or exclude a diagnosis of “obscure” CD limited to the small bowel [9–11].
A meta-analysis reported a pooled odds ratio (OR) for CE of 13.0 (95% CI 3.2–16.3; p < 0.0001) compared with SBFT in detecting small-bowel abnormalities in patients with known or suspected Crohn [13]. The pooled OR for detecting lesions in known or suspected Crohn disease was 5.4 (95% CI 3.0–9.9) for CE compared with enteroclysis. Another meta-analysis focused on 11 prospective comparative studies comparing CE to other modalities for the diagnosis of established or suspected nonstricturing CD [9]. CE was compared to multiple diagnostic modalities (ileoscopy, push enteroscopy, and SBR, including SBFT and enteroclysis, CT enterography, and small bowel MRI) in a total of 228 patients. The yield for CE was significantly higher compared to barium SBR (63% vs. 23%, respectively). Similarly, the yield for CE vs. ileoscopy was 61% and 46%, while that for CE vs. CT was 69% and 30%, respectively. Subset analysis of patients with established CD showed that CE had a higher yield compared to the other modalities. Ongoing issues include the lack of standardization between studies in terms of inclusion and exclusion criteria, as well as the widely variable capsule reading experience. Overall however, CE is now established as more sensitive for small bowel mucosal lesions than other traditional imaging modalities. Moreover, a normal CE examination has a very high negative predictive value, essentially ruling out small bowel CD.
Other techniques for small bowel imaging such as CT enterography (CTE) and MR enterography (MRE) are capable of evaluating bowel wall thickness and enhancement, supporting a diagnosis of CD [14, 15]. In addition to evaluating intestinal wall changes, these techniques can also detect the presence of extraintestinal abnormalities, such as abscess formation. Also, CTE and MRE have been shown to correlate with disease activity [14, 15]. One study compared CE with MRE in 36 adults with known or suspected small bowel CD [16]. Among the 18 patients with known CD, CE detected inflammatory lesions in the proximal and mid small bowel (jejunum and ileum) in 12, compared to only one with MRI (p = 0.016). There was no significant difference in sensitivity between the two studies for disease of the terminal ileum. The authors suggested that CE is better to assess the severity and extent of small bowel inflammation. Another study compared MRE and CE in 27 patients with established and 25 with suspected CD [17]. Among those with established CD, the yield for CE was 93% compared to 79% with MRE. In those with suspected CD, CE was more sensitive and specific (92%/100% vs. 77%/80%, respectively). Another small study in 28 cases used data analysis by consensus diagnosis, comparing CE with CTE, SBFT, and ileocolonoscopy [18]. Although CE had the highest sensitivity (83%) for CD, the specificity was lowest of the four modalities (53%).
Additional prospective studies are required to define the roles of CE vs. CTE or MRE in the diagnostic algorithm for known and suspected CD. A recommended approach, based on available data [19], is illustrated in Fig. 22.2. An economic analysis comparing CE to the traditional modalities for diagnosing CD [20] concluded that CE was a less costly strategy if its diagnostic yield was 64% or greater, based on average diagnostic yields in the literature of 70% for CE and 54% for SBFT and colonoscopy/ileoscopy. The authors suggested that CE may also be less costly as a first-line test in this situation.
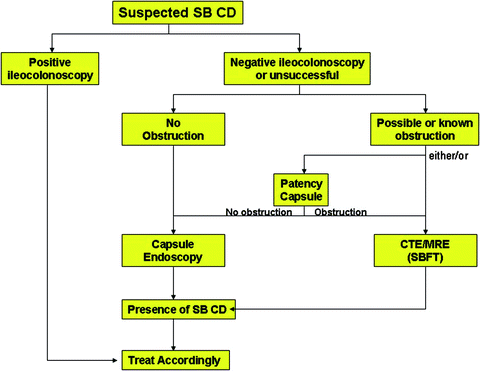

Fig. 22.2
Algorithm for the approach to suspected small bowel Crohn disease (CD). The absence of any mucosal lesions demonstrated by a complete assessment of the small bowel by capsule endoscopy essentially excludes active CD of the small bowel. Patients with symptoms suggestive of or known to have a stenosis should either undergo a patency capsule exam or evaluation by CTE or MRE prior to capsule endoscopy. From: Leighton J, et al.: Inflamm Bowel Dis 2007 (ref. [19]); with permission
Detection of Post-Operative CD Recurrence
Capsule endoscopy may also be used to determine the presence of early postoperative recurrence of CD. A prospective comparison of CE and ileocolonoscopy 6 months after surgery was carried out in 32 adult CD patients, 21 (68%) of whom had recurrent disease [21]. CE was better able to identify proximal small bowel disease. However, ileocolonoscopy was more sensitive overall (90% vs. 62%). Other studies [22, 23] favored CE or CE and abdominal ultrasound. Overall, ileocolonoscopy remains the procedure of choice. However, in view of its noninvasive nature, CE may be considered as an alternative approach in this clinical situation in the pediatric age group. CE may be particularly helpful when the surgical anastomosis is not accessible by endoscopy.
Indeterminate Colitis
Indeterminate colitis (IC), now referred to as IBD of undetermined type (IBD-U), may be defined as a chronic IBD limited to the colon, without clear endoscopic or pathological features diagnostic for either CD or UC. A few pilot studies [24, 25] reported that CE led to a change in diagnosis in 29–40% of patients. One study [25] comparing CE and IBD serological testing in 31 IC patients found equal sensitivity (61%). However, CE was more sensitive than ASCA or Omp-C serology in diagnosing small bowel CD. The authors concluded that CE is superior to IBD serological markers in identifying small bowel CD in IC patients [25]. Another preliminary study looked at the usefulness of CE in IC, Crohn colitis or suspected CD, all with normal SBR [26]. Capsule endoscopy was positive for lesions suggestive of small bowel CD in 16 of 22 patients (73%). The yield was high for those with IC (70%). In a study involving 120 cases of UC and IBD-U, CE revealed findings compatible with CD in 19 cases (15.8%), whereas barium small bowel imaging found just one case [27]. In a pediatric study in 26 cases of IBD-U [28], small bowel lesions typical of Crohn disease were detected by imaging in 7 and by CE in 16 (p < 0.05). Overall, CE shows promise as a diagnostic tool for CD in patients with IBD-U. However, larger prospective studies are needed to confirm the usefulness of CE in this setting.
Use of CE to Evaluate Mucosal Healing
Symptom assessment is a poor indicator of severity and extent of disease. Mucosal healing after 1 year of treatment is predictive of reduced subsequent disease activity and decreased hospitalizations and surgery. Multiple studies have determined that CE can detect subtle mucosal abnormalities of the small bowel. The high diagnostic yield of CE can be useful to evaluate mucosal healing after treatment and thus impact upon disease management and clinical outcomes. Efthyemiou et al. [29] conducted a prospective, multicenter, case-series study. Forty patients with known or suspected CD were included, all with an acute flare-up of nonstricturing, nonpenetrating type of CD. All patients underwent CE prior to the initiation of any treatment. Treatment varied according to the treating physician. For the evaluation of mucosal healing, three endoscopic variables were collected: number of apthous lesions, number of large ulcers, and period of time that any endoscopic lesion was visible (erythema, edema, ulcers). When patients achieved clinical response (after at least a month of treatment) they underwent a second CE, with evaluation of the same parameters. The authors found that the number of large ulcers before and after treatment were 8.3 ± 1.4 and 5 ± 0.8, respectively (mean ± SEM) (mean difference 3.3 ± 1.2, 95% CI 0.8–5.9, p = 0.01).
Another pilot study aimed to determine the efficacy of infliximab in treatment of chronic refractory pouchitis complicated by ileitis, using capsule endoscopy [30]. Wireless capsule endoscopy was repeated at week 10 and the Pouchitis Disease Activity Index score was determined. Clinical remission was achieved in nine of ten patients. At capsule endoscopy and pouch endoscopy, a complete recovery of lesions was observed in eight patients. Although promising, further study of the use of CE to determine mucosal healing of the small bowel after therapy is needed. This concept is particularly appealing for pediatric onset disease, given the noninvasive nature of CE.
Utility of CE in Pediatric Onset IBD
Capsule endoscopy has been approved as a safe and beneficial in the pediatric population [8, 10]. A potential role for CE in suspected CD is substantiated by:
Isolated involvement of the small bowel occurs in ∼30% of CD cases
Normal findings on ileocolonoscopy are not sufficient to exclude CD
Although cross-sectional imaging can detect transmural inflammation, superficial mucosal inflammatory lesions may be missed
Limited studies have been reported on the use of CE in the diagnosis of IBD in children. One trial studied 12 adolescent patients with a clinical suspicion of CD despite negative EGD and colonoscopy [31]. Ileoscopy, achieved in 50% of the patients, was normal in all. Lesions suggestive of CD were identified by CE in 7 of 12 (58%) cases. In our comparative and prospective, self-controlled pediatric trial, 30 patients from 10 to 18 years of age were evaluated for obscure small bowel disease [10]. Lesions consistent with a diagnosis of CD were found only by CE in 10 of 20 (50%) patients suspected of CD, while the diagnosis was formally ruled out in eight patients. Two remaining cases were found to have eosinophilic gastroenteropathy, for an overall diagnostic yield of 60%. Preliminary reports suggest that CE is potentially useful in the evaluation of possible CD among young patients presenting with a protein-losing enteropathy [32] and/or growth failure [33] when other studies are negative.
Specificity of Capsule Findings
No gold standard test exists for the diagnosis of CD. Rather, the diagnosis is based on a compilation of clinical, endoscopic, histological, radiological, and biochemical findings. There is a justifiable concern that normal variant capsule findings in the small bowel may be over-interpreted. A study in adults suggested that up to 13% of normal, asymptomatic individuals can have mucosal breaks and other minor lesions of the small bowel detected by CE [34]. Therefore, CE findings of minor mucosal lesions of the small bowel are alone not sufficient for a diagnosis of CD. Other causes to be considered include celiac disease, infectious, ischemic, autoimmune as well as immunodeficiency-related, allergic and drug-induced etiologies. Nonsteroidal anti-inflammatory drug (NSAID)-induced enteropathy is common and should be excluded, as it is generally conceded that lesions detected by CE in NSAID enteropathy cannot be reliably distinguished from those due to CD. The chronicity of the lesions may assist in the differential diagnosis of CD and NSAID-induced enteropathy [35].
A standard terminology system has been developed along with a CE scoring index for inflammatory lesions such as seen for CD [36]. The parameters that were found to have the necessary inter- and intra-observer consistency were villous edema, mucosal ulcerations, and the presence of stenotic lesions. This “Lewis Score” [36] provides an aid to diagnosis and a measure of mucosal damage:
Combined with other clinical parameters the score could provide a threshold for establishing a positive exam and potentially for the diagnosis of Crohn disease
Provides an objective measure of assessing small bowel disease severity
Assists in determining appropriate patient management
Facilitates communication and standardization for assessing disease states
May help monitor drug therapy effectiveness
In our experience, although scoring the mucosal lesions (villous edema, ulcers, strictures) to compile the “Lewis Score” are not specific for Crohn disease, they do accurately discriminate normal from a positive exam, and gauge the severity of mucosal inflammation (mild, moderate, severe) for each small bowel tertile [36]. Hopefully, such a standardized scoring system will be utilized by clinical investigators carrying out CE so that the data from future trials are standardized and comparable. It will also be important to develop a system for classifying the extent and severity of inflammatory lesions seen on CE in normal individuals and to develop reliable criteria for the diagnosis of CD. Further validation studies in pediatric patients are needed.
Practical Capsule Issues in Pediatric Patients
Capsule Retention
The major contraindication to CE is the presence of a known or suspected gastrointestinal tract obstruction and/or small bowel strictures, because of the risk of capsule retention [37]. The incidence of capsule retention varies widely between reports, from 0.75 to 5% [19]. Most episodes of capsule retention are caused by NSAID, CD, or radiation-induced strictures. In adult patients, tumors are more often implicated as a cause of capsule retention than in pediatrics. Most cases of retention are transitory and remain asymptomatic. However, it may rarely cause symptomatic small bowel obstruction and require endoscopic or surgical removal. To minimize the risk of capsule retention in the small bowel, a careful history should be taken regarding obstructive symptoms. Patients with CD are at a particular risk for stricture formation, and this risk increases with duration and severity of small bowel disease. The rate of capsule retention in patients with suspected CD appears to be quite low. In three reports with a total of 51 patients with suspected CD, no instances of a retained capsule were encountered [38–40]. However, these studies were all conducted in adult patients. In our pediatric prospective trial of CE for suspected CD, capsule retention was seen in 10% (2/20) of cases, despite normal imaging by SBR [10]. In both cases, the capsule passed the unsuspected inflammatory stenosis subsequent to treatment with oral corticosteroids. The rate of capsule retention in patients with known CD is substantially higher, in the range of 4–7% [24, 41]. An example is shown in Fig. 22.3.