Fibroids (leiomyomas, myomas) are an important health care concern because they are the most frequent indication for the performance of hysterectomy, accounting for nearly 240,000 such procedures in the United States (1). In comparison, approximately 30,000 myomectomies were performed that year. Inpatient surgery for fibroids costs $2.1 billion per year in the United States, and the cost of outpatient surgeries, medical and nonmedical costs, and time away from work or family add significantly to these expenditures (2).
Origins of Uterine Fibroids
Fibroids are benign, monoclonal tumors of the smooth muscle cells of the myometrium and contain large aggregations of extracellular matrix composed of collagen, elastin, fibronectin, and proteoglycan (3).
Incidence
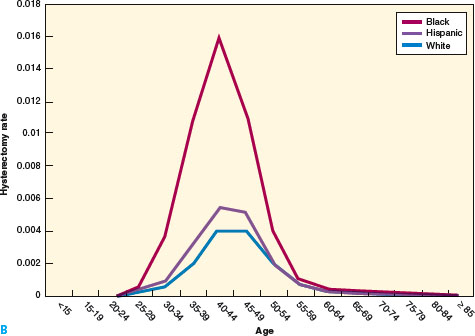
Fibroids are remarkably common. Fine serial sectioning of uteri from 100 consecutive women subjected to hysterectomy discovered fibroids in 77%, some as small as 2 mm (4). A random sampling of women ages 35 to 49, screened by self-report, medical record review, and sonography, found that among African American women by age 35 the incidence of fibroids was 60%, and it was over 80% by age 50 (Fig. 15.1). White women have an incidence of 40% at age 35 and almost 70% by age 50 (5).
Figure 15.1 A: Age-and race-specific incidence of myomectomy, 1997, based on NIS and U.S. Census Bureau estimates. B: Age- and race-specific incidence of hysterectomy for fibroids, 1997, based on NIS and U.S. Census Bureau estimates. (From Health Services/Technology Assessment Tests (HSTAT). Available at http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=hstat1.section.48317.)

Etiology
Although the precise causes of fibroids are unknown, advances have been made in understanding the molecular biology of these benign tumors and their hormonal, genetic, and growth factors (6).
Genetics
Fibroids are monoclonal and about 40% have chromosomal abnormalities that include translocations between chromosomes 12 and 14, deletions of chromosome 7, and trisomy of chromosome12 (7,8). Cellular, atypical, and large fibroids are most likely to show chromosomal abnormalities. The remaining 60% may have as yet undetected mutations. More than 100 genes were found to be up- or down-regulated in fibroid cells (9). Many of these genes appear to regulate cell growth, differentiation, proliferation, and mitogenesis (9). Collagen types I and III are abundant, but collagen fibrils are in disarray, much like the collagen found in keloid formation (10).
Genetic differences between fibroids and leiomyosarcomas indicate that leiomyosarcomas do not result from the malignant degeneration of fibroids. Cluster analysis of 146 genes found that the majority is down-regulated in leiomyosarcomas but not in fibroids or myometrium. Comparative genomic hybridization did not find specific anomalies shared by fibroids and leiomyosarcomas (11).
Hormones
Both estrogen and progesterone appear to promote the development of fibroids. Fibroids are rarely observed before puberty, are most prevalent during the reproductive years, and regress after menopause. Factors that increase overall lifetime exposure to estrogen, such as obesity and early menarche, increase the incidence. Decreased exposure to estrogen found with smoking, exercise, and increased parity is protective (12).
Serum levels of estrogen and progesterone are similar in women with and without clinically detectable fibroids. However, as a result of increased levels of aromatase within fibroids, de novo production of estradiol is higher than in normal myometrium (12). Progesterone is important in the pathogenesis of fibroids, which have increased concentrations of progesterone receptors A and B compared with normal myometrium (13,14). The highest mitotic counts are found in fibroids at the peak of progesterone production (15). Gonadotropin-releasing hormone (GnRH) agonists decrease the size of fibroids, but progestin given concurrently with GnRH prevents a decrease in size (14).
Human fibroid tissue, grafted to immunodeficient mice, increased in size in response to estradiol plus progesterone, but the growth was blocked by the antiprogestin RU486 (16). The volume of grafted fibroid tissue decreased after progesterone withdrawal. Treatment with estradiol alone did not increase the graft size, but did induce expression of progesterone receptors and supported the action of progesterone on the grafts (16).
Growth Factors
Growth factors, proteins, or polypeptides, produced locally by smooth muscle cells and fibroblasts, appear to stimulate fibroid growth primarily by increasing extracellular matrix (6). Many of these growth factors are overexpressed in fibroids and either increase smooth muscle proliferation (transforming growth factor-β [TGF-β, basic fibroblast growth factor [bFGF]), increase DNA synthesis (epidermal growth factor [EGF], platelet-derived growth factor [PDGF]), stimulate synthesis of extracellular matrix (TGF-β), promote mitogenesis (TGF-β, EGF, insulin-like growth factor [IGF], prolactin [PRL]), or promote angiogenesis (bFGF, vascular endothelial growth factor [VEGF]).
Risk Factors
Prospective, longitudinal studies characterize the factors that influence the development of uterine fibroids (4,17,18). Although selection bias may limit epidemiologic studies, the risk factors discussed below are considered.
Age
The incidence of fibroids increases with age, 4.3 per 1,000 woman-years for 25 to 29 year olds and 22.5 for 40 to 44 year olds. African American women develop fibroids at an earlier age than white women (17).
Endogenous Hormonal Factors
Greater exposure to endogenous hormones, as found with early menarche (younger than 10 years of age) increases and late menarche decreases the likelihood of having uterine fibroids (18). Fibroids are smaller, less numerous, and have smaller cells in hysterectomy specimens from postmenopausal women, when endogenous estrogen levels are low (4,19).
Family History
First-degree relatives of women with fibroids have a 2.5 times increased risk of developing fibroids (20). Monozygous twins are reportedly hospitalized for treatment of fibroids more often than heterozygous twins, but these findings may be the result of reporting bias (21).
Ethnicity
African American women have a 2.9 times greater risk of having fibroids than white women, unrelated to other known risk factors (22). African American women have fibroids develop at a younger age and have more numerous, larger, and more symptomatic fibroids (23). It is unclear whether these differences are genetic or result from known differences in circulating estrogen levels, estrogen metabolism, diet, or environmental factors.
Weight
A prospective study found that the risk of fibroids increased 21% with each 10 kg increase in body weight, and with increasing body mass index (BMI) (24). Similar findings were reported in women with greater than 30% body fat (25). Obesity increases conversion of adrenal androgens to estrone and decreases sex hormone binding globulin. The result is an increase in biologically available estrogen, which may explain an increase in fibroid prevalence and/or growth.
Diet
Few studies examined the association between diet and the presence or growth of fibroids (26). A diet rich in beef, other red meat, and ham increased the incidence of fibroids, while a diet rich in green vegetables decreased this risk. These findings are difficult to interpret because calorie and fat intake were not measured.
Exercise
Women in the highest category of physical activity (approximately 7 hours per week) were significantly less likely to have fibroids than women in the lowest category (less than 2 hours per week) (27).
Oral Contraceptives
There is no definite relationship between oral contraceptives and the presence of fibroids. An increased risk of fibroids with oral contraceptive use was reported, but a subsequent study found no increased risk with use or duration of use (28,29). Studies in women with known fibroids who were prescribed oral contraceptives showed no increase in fibroid growth (24,30). The formation of new fibroids does not appear to be influenced by oral contraceptive use (31).
Menopausal Hormone Therapy
For the majority of postmenopausal women with fibroids, hormone therapy will not stimulate fibroid growth. If fibroids do grow, progesterone is likely to be the cause (32). One study evaluated postmenopausal women with fibroids who were given 2 mg of oral estradiol daily and randomized to 2.5 or 5 mg of medroxyprogesterone acetate (MPA) per day (32). One year after starting treatment, 77% of women taking 2.5 mg MPA had either no change or a decrease in fibroids diameters and 23% had a slight increase. However, 50% of women taking 5 mg MPA had an increase in fibroid size (mean diameter increase of 3.2 cm).
Postmenopausal women with fibroids treated with 0.625 of conjugated equine estrogen (CEE) and 5 mg MPA were compared over 3 years to a similar group of women not taking hormone therapy (33). By the end of the third year, only 3 of 34 (8%) treated and 1 of 34 (3%) untreated women had any increase in fibroid volume over baseline (32). Postmenopausal women with known fibroids, followed with sonography, were noted to have an average 0.5 cm increase in the diameter of fibroid after using transdermal estrogen patches plus oral progesterone for 12 months (33). Women taking oral estrogen and progesterone had no increase in fibroid size (34).
Pregnancy
Increasing parity decreases the incidence and number of clinically apparent fibroids (35–37). The remodeling process of the postpartum myometrium, a result of apoptosis and dedifferentiation, may be responsible for the involution of fibroids (38). Another theory postulates that the vessels supplying fibroids regress during involution of the uterus, depriving fibroids of their source of nutrition (39).
Smoking
Smoking reduces the incidence of fibroids. Reduced conversion of androgens to estrone, caused by inhibition of aromatase by nicotine, increased 2-hydroxylation of estradiol, and stimulation of higher levels of sex hormone–binding globulin (SHBG) decrease bioavailability of estrogen (40–42).
Tissue Injury
Cellular injury or inflammation resulting from an environmental agent, infection, or hypoxia was proposed as a mechanism for initiation of fibroid formation (43). Repetitive tissue injury to the endometrium and endothelium might promote the development of monoclonal smooth muscle proliferations in the muscular wall. Frequent mucosal injury with stromal repair (menstruation) may release growth factors that promote the high frequency of uterine fibroids (43).
No increased incidence was found in women with prior sexually transmitted infections, prior intrauterine device (IUD) use or prior talc exposure (35). Herpes simplex virus (HSV) I or II, cytomegalovirus (CMV), Epstein-Barr virus (EBV), and chlamydia were not found in fibroids.
Symptoms
Fibroids are almost never associated with mortality, but they may cause morbidity and significantly affect quality of life (44). Women who have hysterectomies because of fibroid-related symptoms have significantly worse scores on SF-36 quality-of-life questionnaires than women diagnosed with hypertension, heart disease, chronic lung disease, or arthritis (44).
Of 116 women with fibroids larger than 5 cm on sonographic examination and uterine size greater than 12 cm on pelvic examination, 42% were satisfied with their initial level of symptoms, including stress, bleeding, and pain (45). Most of the 48 women who chose to have treatment within 1 year were more likely to have higher scores on bleeding and pain scales and be more concerned about their symptoms. Most women chose myomectomy (n = 20), hysterectomy (n = 15), or hysteroscopic myomectomy (n = 4), and symptoms scores improved markedly during the 7.5 months (mean) of follow-up.
Abnormal Bleeding
The association of fibroids with menorrhagia is not clearly established. Therefore, other possible etiologies, including coagulopathies such as von Willebrand’s disease, should be considered in a woman with heavy menstrual bleeding (46).
A random sample of women aged 35 to 49 was evaluated by self-reported bleeding patterns and by abdominal and transvaginal sonography to determine the presence, size, and location of fibroids (47). Of the 878 women screened, 564 (64%) had fibroids and 314 (36%) did not. Forty-six percent of women with fibroids reported, “gushing blood” during menstrual periods, compared with 28% without fibroids. Gushing blood and length of periods were related to the size of fibroids but not to the presence of submucous fibroids or multiple fibroids.
Another study found that women with fibroids used 7.5 pads or tampons on the heaviest day of bleeding compared with 6.1 pads or tampons used by women without fibroids (48). Women with fibroids larger than 5 cm had slightly more gushing and used about three more pads or tampons on the heaviest day of bleeding than women with smaller fibroids.
Pain
Women with fibroids are only slightly more likely to experience pelvic pain than women without fibroids. Transvaginal sonography was performed on a population-based cohort of 635 non-care-seeking women with an intact uterus to determine the presence of uterine fibroids (49). Dyspareunia, dysmenorrhea, or noncyclic pelvic pain was measured by visual analog scales. The 96 women found to have fibroids were only slightly more likely to report moderate or severe dyspareunia or noncyclic pelvic pain and had no higher incidence of moderate or severe dysmenorrhea than women without fibroids. Neither the number nor the total volume of fibroids was related to pain. However, women who present for clinical evaluation for fibroid-associated pain may be different from those in the general population (49).
Fibroid degeneration may cause pelvic pain. As fibroids enlarge, they may outgrow their blood supply, with resulting cell death (50). Types of degeneration determined both grossly and microscopically include hyaline degeneration, calcification, cystic degeneration, and hemorrhagic degeneration. The type of degeneration appears to be unrelated to the clinical symptoms (50). Pain from fibroid degeneration is often successfully treated with analgesics and observation. Torsion of a pedunculated subserosal fibroid may produce acute pelvic pain that requires surgical intervention (51).
Urinary Symptoms
Fibroids may cause urinary symptoms, although few studies examined this association. Following uterine artery embolization with a 35% reduction in mean uterine volume, frequency and urgency were greatly or moderately improved in 68% of women, slightly improved in 18%, and unchanged or worse in only 14% (52). This finding suggests that increased uterine volume associated with fibroids is related to urinary symptoms.
Fourteen women with large fibroids and urinary symptoms were given six monthly injections of GnRH agonist (GnRH-a) with a resulting 55% decrease in uterine volume (53). Following therapy, urinary frequency, nocturia, and urgency decreased. There were no changes in urge or stress incontinence as measured by symptoms or urodynamic studies. It is not clear whether these findings are related to a decrease in uterine volume or to other effects of GnRH treatment.
Natural History of Fibroids
Most fibroids grow slowly. A prospective, longitudinal study of 72 premenopausal women (38 black, 34 white) using computer analysis of serial MRI found that the median growth rate was 9% over 12 months (17). However, multiple fibroids in the same individual were found to have highly variable growth rates, suggesting that growth results from factors other than hormone levels. After age 35, growth rates declined with age for white women but not for black women, which likely explains the increased fibroid-related symptoms noted in black women. Seven percent of fibroids regressed over the study period. Continued follow-up of these women is planned and should provide a better understanding of this important issue.
Rapid Fibroid Growth
In premenopausal women, “rapid uterine growth” almost never indicates presence of uterine sarcoma. One study found only 1 sarcoma among 371 (0.26%) women operated on for rapid growth of presumed fibroids (54). No sarcomas were found in the 198 women who had a 6-week increase in uterine size over 1 year, which is the definition of rapid growth that was used in the past.
Uterine Sarcoma
Women found to have uterine sarcoma are often clinically suspected of having a pelvic malignancy (54,55). Women with pain and bleeding and who are closer to menopause or postmenopausal may have a rare sarcoma. Of nine women found to have uterine sarcomas, all were postmenopausal and eight were admitted with abdominal pain and vaginal bleeding (55). All eight had presumed gynecologic malignancies: uterine sarcoma in four, endometrial carcinoma in three, and ovarian cancer in one. One additional woman had surgery for prolapse and a sarcoma was found incidentally (55). Between 1989 and 1999, the Surveillance, Epidemiology, and End Results (SEER) database reported 2,098 women with uterine sarcomas with an average age of 63 years, whereas a literature review found a mean age of 36 years in women subjected to myomectomy (54,56).
Diagnosis
Pelvic Examination
Clinically significant subserosal and intramural fibroids can usually be diagnosed by pelvic examination based on findings of an enlarged, irregularly shaped, firm and nontender uterus (57). Uterine size assessed by bimanual examination, even for most women with BMI greater than 30, correlates well with uterine size and weight at pathological examination (58). Routine sonographic examination is not necessary when the diagnosis is almost certain. However, a definite diagnosis of submucous fibroids often requires saline-infusion sonography, hysteroscopy, or magnetic resonance imaging (MRI) (59).
Fibroid Location
The FIGO fibroid classification system categorizes submucous, intramural, subserosal, and transmural fibroids.
Transmural fibroids are categorized by their relationship to both the endometrial and the serosal surfaces, with the endometrial relationship noted first, e.g., type 2–3 (Table 15.1; Fig 15.2) (60).
Table 15.1 FIGO Leiomyoma Classification System
| SM – Submucosal | 0 | Pedunculated intracavitary |
| 1 | <50 intramural | |
| 2 | ≥50 intramural | |
| O – Other | 3 | Contacts endometrium; 100 intramural |
| 4 | Intramural | |
| 5 | Subserosal ≥50 intramural | |
| 6 | Subserosal <50 intramural | |
| 7 | Subserosal pedunculated | |
| 8 | Other (specify e.g. cervical, parasitic) | |
| Hybrid leiomyomas (impact both endometrium and serosa) | Two numbers are listed separated by a hyphen. By convention, the first refers to the relationship with the endometrium while the second refers to the relationship to the serosa. One example is below | |
| 2–5 | Submucosal and subserosal, each with less than half the diameter in the endometrial and peritoneal cavities, respectively. |
Figure 15.2 FIGO Leiomyoma Classification System.

Imaging
For symptomatic women, consideration of medical therapy, noninvasive procedures, or surgery often depends on an accurate assessment of the size, number, and position of fibroids. Transvaginal sonography (TVS), saline-infusion sonography (SIS), hysteroscopy, and MRI were all performed on 106 women scheduled for hysterectomy and the findings were compared to pathologic examination (59). Submucous fibroids were best identified with MRI (100% sensitivity, 91% specificity). Identification was about equal with transvaginal sonography (sensitivity 83%, specificity 90%), saline-infusion sonography (sensitivity 90%, specificity 89%), and hysteroscopy (sensitivity 82%, specificity 87%). MRI is not technique dependent and has low interobserver variability for diagnosis of submucous fibroids, intramural fibroids, and adenomyosis when compared with TVS, SIS, and hysteroscopy (61,62).
The presence of adenomyosis is associated with junctional zone thickness of more than 15 mm (or 12 mm in a nonuniform junctional zone). Focal, not well-demarcated, and high or low intensity areas in the myometrium correlate with adenomyosis (63).
MRI allows evaluation of the number, size, and position of submucous, intramural, and subserosal fibroids and can evaluate their proximity to the bladder, rectum, and endometrial cavity. MRI helps define what can be expected at surgery and might help the surgeon avoid missing fibroids during surgery (64). For women who wish to preserve fertility, MRI to document location and position relative to the endomyometrium may be helpful prior to hysteroscopic, laparoscopic, or abdominal myomectomy.
Sonography is the most readily available and least costly imaging technique to differentiate fibroids from other pelvic pathology and is reasonably reliable for evaluation of uterine volume less than 375 cc and containing four or fewer fibroids (61). Sonographic appearance of fibroids can be variable, but often they appear as symmetrical, well-defined, hypoechoic and heterogenous masses. Areas of calcification or hemorrhage may appear hyperechoic, while cystic degeneration may appear anechoic. SIS utilizes saline inserted into the uterine cavity to provide contrast and better defines submucous fibroids (61).
Imaging of Uterine Sarcomas
The preoperative diagnosis of leiomyosarcoma may be possible. Diagnosis with total serum lactate dehydrogenase (LDH) and LDH isoenzyme 3 measurements along with gadolinium-enhanced diethylenetriamine penta-acetic acid (Gd-DTPA) dynamic MRI was reported to be highly accurate (65). MRI images are taken during the arterial phase, between 40 and 60 seconds after infusion of gadolinium. Sarcomas have increased vascularity and show increased enhancement with gadolinium, while degenerating fibroids have decreased perfusion and exhibit decreased enhancement. Using LDH measurements and Gd-DTPA, a study of 87 women with uterine fibroids, 10 women with leiomyosarcomas, and 130 women with degenerating fibroids reported 100% specificity, 100% positive predictive value, 100% negative predictive value, and 100% diagnostic accuracy for leiomyosarcoma (Fig. 15.3).
Fertility
The presence of submucous fibroids decreases fertility rates and removing them increases fertility rates. Subserosal fibroids do not affect fertility rates but removing them does increase fertility. Intramural fibroids may slightly decrease fertility, but removal does not increase fertility (66). A meta-analysis of the effect of fibroids on fertility and the effect of myomectomy on fertility found that submucous fibroids that distort the uterine cavity appear to decrease fertility, with ongoing pregnancy/live birth rates decreased by about 70% (relative risk [RR] 0.32; 95% confidence interval [CI], 0.12–0.85) (66). Resection of submucous fibroids slightly increased fertility relative to infertile controls without fibroids (ongoing pregnancy/live birth rate, RR 1.13; 95% CI, 0.96–1.33).
Analysis of studies that routinely used hysteroscopy to confirm clear nondistortion of the cavity by intramural fibroids found ongoing pregnancy/live birth rates were not significantly different compared to controls (RR 0.73; 95% CI, 0.38–1.40) (66). Importantly, removal of intramural or subserosal fibroids did not improve ongoing pregnancy/live birth rates (RR 1.67; 95% CI, 0.75–3.72).
Myomectomy may involve operative and anesthetic risks, risks of infection or postoperative adhesions, a slight risk of uterine rupture during pregnancy, an increased likelihood that a cesarean section will be recommended for delivery, and the expense of surgery and time for recovery. Therefore, until intramural fibroids are shown to decrease and myomectomy to increase fertility rates, surgery should be undertaken with reluctance (66). Randomized studies are needed to clarify the relative risks and benefits of surgical intervention.
Fibroids and Pregnancy
Incidence of Fibroids during Pregnancy
The prevalence of fibroids among pregnant women is 18% in African American women, 8% in white women, and 10% in Hispanic women, based on first trimester sonography (67). Mean size of the fibroids was 2.5 cm. Clinical examination detects 42% of fibroids greater than 5 cm during pregnancy, but only 12.5% when they are less than 5 cm (68).
Figure 15.3 MR images. A: Degenerating fibroid. Left to right. Preenhanced T1 image, T2 image, and no enhancement on T1 Gd-DPTA at 60 seconds. B: Leiomyosarcoma. Left to right, preenhanced T1 image, T2 image (arrow to dorsal part of tumor), and enhancement of dorsal part of tumor (arrow) on T1 Gd-DPTA at 60 seconds. (From Goto A, Takeuchi S, Sugimura K, et al. Usefulness of Gd-DTPA contrast-enhanced dynamic MRI and serum determination of LDH and its isozymes in the differential diagnosis of leiomyosarcoma from degenerated leiomyoma of the uterus. Int J Gynecol Cancer 2002;12:354–361.)

Effect of Pregnancy on Fibroids
Most fibroids do not increase in size during pregnancy. Pregnancy has a variable, and unpredictable, effect on fibroid growth, likely dependent on individual differences in fibroid gene expression, circulating growth factors, and fibroid-localized receptors (68,69). A prospective study of 36 pregnant women with a single fibroid discovered during routine first trimester sonographic screening and examined by sonography at 2- to 4-week intervals found that 69% of the women had no increase in fibroid volume throughout pregnancy (69). In the 31% of women noted to have an increase in volume, the greatest increase occurred before the 10th week of gestation. There was no relationship between initial fibroid volume and fibroid growth during gestational periods. A reduction in fibroid size toward baseline measurements was observed 4 weeks after delivery.
Fibroid Degeneration during Pregnancy
In women noted to have fibroids during pregnancy, clinical symptoms and sonographic evidence of fibroid degeneration occurs in about 5% (70). Among 113 women followed during pregnancy with serial sonography, 10 (9%) developed anechoic spaces or coarse heterogenous patterns consistent with fibroid degeneration. Seven of 10 women had severe abdominal pain requiring hospitalization, consistent with clinical symptoms of degeneration. No sonographic changes were noted in the other 103 women, and only 11.7% had similar abdominal pain. A small study of women with fibroid-associated pain during pregnancy found use of ibuprofen shortened the hospital stay and decreased the rate of readmission (71).
Influence of Fibroids on Pregnancy
Very rarely does the presence of a fibroid during pregnancy lead to an unfavorable outcome. Research was conducted on large populations of pregnant women examined with routine second trimester sonography with follow-up care and delivery at the same institution (72,73). In a study of 12,600 pregnant women, the outcomes of 167 women with fibroids were no different with regard to the incidence of preterm delivery, premature rupture of membranes, fetal growth restriction, placenta previa, placental abruption, postpartum hemorrhage, or retained placenta (72). Cesarean section was more common among women with fibroids (23% vs. 12%).
The other study of 15,104 pregnancies, including 401 women with fibroids, found no increased risk of premature rupture of membranes, operative vaginal delivery, chorioamnionitis, or endometritis (73). However, there were increased risks of preterm delivery (19.2% vs. 12.7%), placenta previa (3.5% vs. 1.8%), and postpartum hemorrhage (8.3% vs. 2.9%). Cesarean section was again more common (49.1% vs. 21.4%).
Fetal injury attributed to mechanical compression by fibroids is uncommon. A search of the PubMed database from 1980 to 2010 revealed one case of fetal head anomalies with fetal growth restriction, one case of a postural deformity, one case of a limb reduction, and one case of fetal head deformation with torticollis (74—75).
Any decision to perform a myomectomy in order to prevent problems during pregnancy should take into account the risks of surgery, anesthesia, postoperative adhesions, and an increased likelihood of subsequent cesarean delivery, along with concerns about discomfort, expense, and time away from work or family.
Rupture of Myomectomy Scar during Pregnancy
Following abdominal myomectomy, uterine rupture during pregnancy appears to be a rare event. Two studies comprising 236,454 deliveries reported 209 instances of uterine rupture, with only 4 cases attributable to prior myomectomy (78,79). Because the number of women who had a previous myomectomy was not known, the incidence of rupture in these studies could not be determined. However, a retrospective study of 412 women who had abdominal myomectomies reported only one woman with uterine rupture (0.2%) (80).
Operative techniques, instruments, and energy sources used during laparoscopic myomectomy may differ from those employed during laparotomy. A study of 19 published and unpublished cases of uterine rupture during pregnancy following laparoscopic myomectomy found that almost all cases involved deviations from standard surgical technique as described for abdominal myomectomy (81). In seven cases, the uterine defect was not repaired; in three cases it was repaired with a single suture; in four cases it was repaired with only one layer of suture; and in one case only the serosa was closed. In only three cases was a multilayered closure employed. In 16 of the cases, monopolar or bipolar energy was used for hemostasis.
Although definite conclusions and recommendations regarding appropriate technique for laparoscopic myomectomy must await proper study of myometrial wound healing, it appears prudent for surgeons to adhere to time-tested techniques developed for abdominal myomectomy, including multilayered closure of myometrium (for other than superficial uterine defects) and limited use of electro-surgery for hemostasis. Yet, even with ideal surgical technique, individual wound healing characteristics may predispose to uterine rupture.
Treatment
The development of new treatments for fibroids is slow, perhaps because many women with fibroids are asymptomatic, fibroids are benign, and mortality is very low (82). If offered hysterectomy as a first, and sometimes only, treatment option, some women choose to accommodate to symptoms and stop seeking treatment. This may lead physicians to underestimate the true impact of the condition, despite the fact that women who have hysterectomies as a result of fibroid-related symptoms have significantly worse scores on SF-36 quality-of-life questionnaires than women diagnosed with hypertension, heart disease, chronic lung disease, or arthritis (44).
After an exhaustive review of the medical literature published between 1975 and 2000, with evaluation of 637 relevant articles and careful study of 200 articles, the authors found no satisfactory answers to fundamental question about fibroid treatments (83). Women and their physicians need information on which to base decisions regarding possible treatments.
This section summarizes the literature regarding the management of fibroids. Treatment options include observation, medical therapy, hysteroscopic myomectomy, laparoscopic myomectomy, hysterectomy, uterine artery embolization, and focused ultrasound.
Watchful Waiting
Not having treatment for fibroids rarely results in harm, except for women with severe anemia from fibroid-related menorrhagia or hydronephrosis from ureteric obstruction from an massively enlarged fibroid uterus. Predicting future fibroid growth or onset of new symptoms is not possible (84). During observation, the average fibroid volume increases 9% per year with a range of −25% to +138% (84). A nonrandomized study of women with uterine size 8 weeks or greater who chose watchful waiting found that 77% of women had no significant changes in the self-reported amount of bleeding, pain, or degree of bothersome symptoms at the end of 1 year (85). Furthermore, mental health, general health, and activity indexes were also unchanged. Of the 106 women who initially chose watchful waiting, 23% opted for hysterectomy during the course of the year.
Therefore, for women who are mildly or moderately symptomatic with fibroids, watchful waiting may allow treatment to be deferred, perhaps indefinitely. As women approach menopause, watchful waiting may be considered, because there is limited time to develop new symptoms and after menopause, bleeding stops and fibroids decrease in size (19). Although not specifically studied, the incidence of hysterectomy for fibroids declines considerably after menopause, suggesting that there is a significant decline in symptoms.
Medical Therapy
Nonsteroidal Anti-inflammatory Medication
Nonsteroidal anti-inflammatory drugs (NSAIDs) were not shown to be effective for the treatment of menorrhagia in women with fibroids. A placebo-controlled, double-blind study of 25 women with menorrhagia, 11 of whom also had fibroids, found a 36% decrease in blood loss among women with idiopathic menorrhagia, but no decrease in women with fibroids. No other studies examined this treatment (86).
Gonadotropin-Releasing Hormone Agonists
Treatment with GnRH-a decreases uterine volume, fibroid volume, and bleeding. However, the benefits of GnRH-a are limited by side effects and risks associated with long-term use (87,88). Monthly GnRH-a given for 6 months reduced fibroid volume by 30% and total uterine volume by 35% (87). Reduction in uterine size occurs mostly within the first 3 months of treatment (88). Menorrhagia responds well to GnRH-a; 37 of 38 women had resolution by 6 months. Following discontinuation of GnRH-a, menses returns in 4 to 8 weeks and uterine size returns to pretreatment levels within 4 to 6 months (89). In this study 64% of women remained asymptomatic 8 to 12 months after treatment.
Side effects occur in 95% of women treated with GnRH-a (89). Seventy-eight percent experience hot flushes, 32% vaginal dryness, and 55% have transient frontal headaches. However, during 6 months of treatment only 8% of women discontinued GnRH-a because of the side effects. Arthralgia, myalgia, insomnia, edema, emotional lability, depression, and decreased libido are reported. The hypoestrogenic state induced by GnRH-a causes significant bone loss after 6 months of therapy (90).
In an effort to reduce side effects, inhibit bone loss, and allow longer-term use of GnRH-a, low doses of estrogen and progestins may be added while continuing GnRH-a. However, a study of long-term use of GnRH-a over 6 years found a wide range of reduction in bone density among women and no difference in bone loss between groups given estrogen and progestin versus those treated with GnRH-a alone (91).
Gonadotropin-Releasing Hormone Agonist as Temporary Treatment for Perimenopausal Women
Women in late perimenopause who are symptomatic from uterine fibroids may consider short-term use of GnRH-a. Thirty-four perimenopausal women with symptomatic fibroids were treated with GnRH-a for 6 months, 12 of whom required repeat treatment 6 months after discontinuation of the medication (92). Thirty-one women avoided surgery; 15 of the women went into natural menopause. Although not specifically studied, add-back therapy might be considered in this setting.
Gonadotropin-Releasing Hormone Antagonist
The immediate suppression of endogenous GnRH by daily subcutaneous injection of the GnRH antagonist ganirelix results in a 29% reduction in fibroid volume within 3 weeks (93). Treatment is accompanied by hypoestrogenic symptoms. When long-acting compounds are available, a GnRH antagonist might be considered for medical treatment prior to surgery.
Progesterone-Mediated Medical Treatment
The reduction in uterine size following treatment with the progesterone-blocking drug mifepristone is similar to that found with GnRH-a (94). A prospective, randomized, controlled trial of mifepristone treatment found a 48% decrease in mean uterine volume after 6 months (95). Mifepristone blocks progesterone, and the unopposed exposure of the endometrium to estrogen may lead to endometrial hyperplasia. A systematic review found endometrial hyperplasia in 10 of 36 (28%) women screened with endometrial biopsies (96).
Progesterone-Releasing Intrauterine Device
The levonorgestrel-releasing intrauterine system (LNG-IUS) may be reasonable treatment for selected women with fibroid-associated menorrhagia. In women with fibroids, uterine size no larger than 12 weeks, and a normal uterine cavity, LNG-IUS substantially reduces menstrual bleeding (97). Twenty-two of 26 (85%) women with documented fibroid-related menorrhagia returned to normal bleeding within 3 months. By 12 months, 27 of 67 (40%) women had amenorrhea and 66 women had hemoglobin levels above 12 g/dL.
One study examined 32 women with at least one fibroid less than 5 cm in diameter and less than 50% of the tumor volume projecting into the endometrial cavity (type II) who had insertion of an LNG-IUS (98). After 12 months, mean estimated blood loss, measured by pictorial blood loss assessment, decreased from 392 to 37 mL with an associated increase in hemoglobin levels. There was no change in uterine volume over the course of the study. Some studies show that LNG-IUS expulsion rates are higher in women with fibroids than in women without fibroids (99).
Alternative Medicine Treatment
A nonrandomized, nonblinded study compared fibroid growth in 37 women treated with Chinese medicine, body therapy, and guided imagery to 37 controls treated with nonsteroidal anti-inflammatory medications, progestins, or oral contraceptive pills (100). After 6 months, sonographic evaluation demonstrated that fibroids stopped growing or shrank in 22 of 37 (59%) women treated with Chinese medicine compared to 3 of 37 (8%) controls. Although symptoms responded equally well in both groups, satisfaction was higher in the Chinese medicine group. Participants actively sought alternative therapy, however, and assessment of satisfaction may reflect selection bias.
An uncontrolled study reported treatment of 110 women with fibroids smaller than 10 cm with the Chinese herbal medicine kuei-chih-fu-ling-wan for at least 12 weeks (101). Clinical and sonographic evaluation found complete resolution of fibroids in 19% of women, a decrease in size in 43%, no change in 34%, and an increase in 4%. Menorrhagia improved in 60 of 63 (95%) of women and dysmenorrhea improved in 48 of 51 (94%). Fifteen of the 110 (14%) women chose to have a hysterectomy during the 4 years of the study.
Surgical Treatment Options
Surgical treatment options currently include abdominal myomectomy, laparoscopic myomectomy, hysteroscopic myomectomy, endometrial ablation, and abdominal, vaginal, or laparoscopic hysterectomy.
Serious medical conditions, such as severe anemia or ureteral obstruction, often need to be addressed surgically. Pain from fibroid degeneration is usually successfully treated with analgesics until symptoms resolve, but if severe the patient may opt for surgery. Torsion of a pedunculated subserosal fibroid may produce acute pain that requires surgical intervention. Surgical intervention may be indicated in women with fibroids associated with menorrhagia, pelvic pain or pressure, urinary frequency, or incontinence that compromises quality of life (102).
Abdominal myomectomy was long employed as a conservative treatment for uterine fibroids, and much of the literature predates the use of prospective, randomized controlled trials. Although myomectomy is stated to relieve symptoms in 80% of women, there is scant literature documenting its efficacy and many large series have not reported data for relief of symptoms following surgery (102–104). A prospective, nonrandomized study comparing myomectomy with uterine artery embolization did report that 75% of women in the myomectomy group had a significant decrease in symptom scores after 6 months (105).
Back pain may, on occasion, be related to the presence of fibroids, but other possible causes should be considered. Inability to evaluate the ovaries on pelvic examination is not an indication for surgery (106). There is no evidence that pelvic examination increases early detection or decreases the mortality related to ovarian cancer, and sonography can be used to evaluate the adnexa should symptoms develop.
Treating Preoperative Anemia
Recombinant Erythropoietin
Severe anemia can be rapidly corrected using recombinant forms of erythropoietin and iron supplementation. Erythropoietin alfa and epoetin are commonly used to increase preoperative hemoglobin concentrations in cardiac, orthopaedic, and neurologic surgery. A randomized study showed that use of epoetin 250 IU/kg (approximately 15,000 U) per week for 3 weeks prior to elective orthopaedic or cardiac surgery increased the hemoglobin concentrations by 1.6 g/dL and significantly reduced transfusion rates when compared to controls (107). No side effects were experienced. A prospective, nonrandomized study of epoetin given preoperatively found a significant increase in hemoglobin concentrations prior to, and following, gynecologic surgery (108). For best results, iron stores should be increased with supplemental iron. Vitamin C, 1,000 IU per day, increases iron absorption in the intestines.
Gonadotropin-Releasing Hormone Agonist
GnRH-a may be used preoperatively to mitigate abnormal bleeding, with a resultant increase of hemoglobin concentration. Women with fibroids and initial mean hemoglobin concentrations of 10.2 g were randomized preoperatively to GnRH-a plus oral iron or placebo plus oral iron (109). After 12 weeks, 74% of the women treated with GnRH-a and iron had hemoglobins greater than 12 g compared with 46% of the women treated with iron alone.
A Cochrane review found that women with fibroids treated preoperatively with 3 to 4 months of GnRH-a had improved preoperative hemoglobins (110). Although operative blood loss was less for abdominal myomectomy patients treated with GnRH, there was no significant difference in transfusion rates compared with untreated women.
Abdominal Myomectomy
Myomectomy should be considered a safe alternative to hysterectomy. Victor Bonney, an early advocate of abdominal myomectomy, stated in 1931 that “The restoration and maintenance of physiologic function is, or should be, the ultimate goal of surgical treatment.” Case-controlled studies suggest that there may be less risk of intraoperative injury with myomectomy when compared with hysterectomy (111). A retrospective review of 197 women who had myomectomies and 197 women who underwent hysterectomies with similar uterine size (14 versus 15 weeks) found operating times were longer in the myomectomy group (200 versus 175 minutes), but estimated blood loss was greater in the hysterectomy group (227 versus 484 mL) (111). The risks of hemorrhage, febrile morbidity, unintended surgical procedure, life-threatening events, and rehospitalization were no different between groups. However, 26 (13%) women in the hysterectomy group suffered complications, including 1 bladder injury, 1 ureteral injury, 3 bowel injuries, 8 women with ileus, and 6 women with pelvic abscesses. In contrast, complications occurred in 11 (5%) of the myomectomy patients, including 1 bladder injury, 2 women with reoperation for small bowel obstruction, and 6 women with ileus.
Myomectomy may be considered even for those women who have large uterine fibroids and wish to retain their uterus. A study of 91 women with uterine size larger than 16 cm (range 16–36 cm) reported one bowel injury, one bladder injury, and one reoperation for bowel obstruction, but no women had conversion to hysterectomy (112). The cell saver, which is a devise used to collect blood intraoperatively and reinfuse, was used in 70 women, and only 7 required homologous blood transfusion. A retrospective cohort study compared 89 women having abdominal hysterectomy for fibroids (mean uterine size 15 cm) to abdominal myomectomy in 103 women (mean uterine size 12 cm) (113). Although selection bias was likely, the hysterectomy group suffered two ureteral, one bladder, one bowel, and one nerve injury and two reoperations for bowel obstruction, while there were no visceral injuries in the myomectomy group.
Cesarean Section and Concurrent Myomectomy
In carefully selected women, myomectomy may be safely accomplished at the time of cesarean section by experienced surgeons. One series reported 25 women with removal of 84 fibroids (2 to 10 cm) at the time of cesarean section without the need for cesarean hysterectomy (114). Estimated blood loss was 876 mL (range 400 to 1,700 mL) and five women required blood transfusion. Another study compared 111 women who had myomectomy at the time of cesarean section with 257 women with fibroids who were not subjected to myomectomy during cesarean section (115). Only one of the women in the myomectomy group required transfusion and none required hysterectomy or embolization. There were no differences in mean operative times, incidence of fever, or length of hospital stay between the two groups. Although the cases were likely selected carefully, the authors concluded that, in experienced hands, myomectomy might be safely performed in selected women during cesarean section.
Surgical Technique for Abdominal Myomectomy
Managing Blood Loss
Available surgical techniques allow safe removal of even large fibroids. Use of tourniquets or vasoconstrictive agents may be used to limit blood loss. Vasopressin, an antidiuretic hormone, causes constriction of smooth muscle in the walls of capillaries, small arterioles and venules. Synthetic vasopressin (Pitressin, Parke-Davis, NJ) decreases blood loss during myomectomy and in a prospective, randomized study was as effective as mechanical occlusion of the uterine and ovarian vessels (116,117). Rare cases of bradycardia and cardiovascular collapse were reported; intravascular injection should be carefully avoided and patients should be carefully monitored (118). The use of vasopressin to decrease blood loss during myomectomy is an off-label use of this drug.
Cell savers may be considered for use during myomectomy. Use of the cell saver avoids the risks of infection and transfusion reaction, the oxygen transport capacity of salvaged red blood cells is equal to or better than stored allogeneic red cells, and the survival of red blood cells appears to be at least as good as transfused allogeneic red cells (119). The device suctions blood from the operative field, mixes it with heparinized saline, and stores the blood in a canister. If the patient requires blood reinfusion, the stored blood is washed with saline, filtered, centrifuged to a hematocrit of approximately 50%, and given back to the patient intravenously. Consequently, the need for preoperative autologous blood donation or heterologous blood transfusion often can be avoided (120). In a study of 92 women who had myomectomy for uterine size greater than 16 cm the cell saver was used for 70 women with a mean volume of reinfused packed red blood cells of 355 mL (121).
The cost of using a cell saver compared with donation of autologous blood was not studied for abdominal myomectomy. However, economic models suggest it is cost effective (121). Most hospitals charge a minimal fee for having the cell saver available “on-call” and charge an additional fee if it is used. Assuming that most women who donate autologous blood prior to myomectomy do not require blood transfusion, availability of the cell saver should spare many women the time and expense of donating, storing, and processing autologous blood. For a cohort of women, the cost of using the cell saver should, therefore, be significantly lower than the cost of autologous blood.
When heavy bleeding is anticipated or if copious bleeding is encountered, ligation of both uterine arteries can be performed (122). Uterine artery embolization was used successfully to control bleeding at the time of, or following, myomectomy (123). Because the uterine arteries recanulate, future fertility should not be compromised. These techniques often obviate the need for hysterectomy.
Uterine incisions can be made either vertically or transversely, because fibroids distort normal vascular architecture, making attempts to avoid the arcuate vessels impossible (124). However, careful planning and placement of uterine incisions can avoid inadvertent extension of the incision to the uterine cornua or ascending uterine vessels.
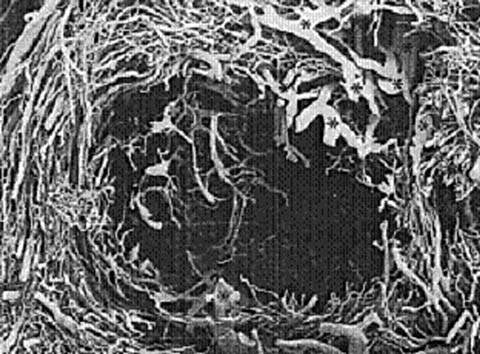
Based on vascular corrosion casting and examination by electron microscopy, fibroids are completely surrounded by a dense blood supply and no distinct vascular pedicle exists at the base of the fibroid (125) (Fig. 15.4). Extending the uterine incisions through the myometrium and entire pseudocapsule until the fibroid is clearly distinguished identifies a less vascular surgical plane, which is deeper than commonly recognized.
Figure 15.4 Corrosion casting of fibroid vessels.