CHAPTER 32 Uterine fibroids
Introduction
There continues to be an appalling paucity of research on this tumour and its treatment. This is of particular concern given that it occurs in more than two-thirds of women at some stage of their lives, and is the leading indication for the most common major gynaecological operation in women — hysterectomy. The annual cost of inpatient surgery for fibroids is estimated to be $US2.1 billion in the USA (Parker 2007), with additional indirect costs from outpatient treatment and lost time at work.
Incidence
The true incidence of uterine fibroids in the general female population is unknown. This is because most studies on fibroids have only included women with symptoms or women having treatments such as hysterectomy. Given that up to 50% of fibroids are asymptomatic (Buttram and Reiter 1981), the former approach can be expected to underestimate the true population incidence. However, studies of incidence based on histological examination of hysterectomy specimens may similarly overestimate the incidence of fibroids, as they concentrate the worst cases of menorrhagia where medical treatments have failed. Cramer and Patel (1990) found that 77% of hysterectomy specimens had fibroids on histology, although fibroids were only detected in 33% of patients clinically and 50% of patients by ultrasound examination.
The lifetime risk of a woman over 45 years of age developing fibroids is greater than 60%, with reported incidences in different studies varying from 30% to 70% for premenopausal women (Okolo 2008).
Aetiology
The precise aetiology of fibroids remains unknown. It is known that each fibroid is monoclonal, developing from a single cell. Electrophoresis patterns of glucose-6-phosphate dehydrogenase isoenzyme (A or B) demonstrated this in early studies (Townsend et al 1970). Later, polymerase chain reaction experiments confirmed monoclonality by the pattern of inactivation of the X-chromosome-linked phosphoglycerokinase gene (Hashimoto et al 1995). In the development of fibroid tumours, some form of initiation event must transform a normal myometrial cell into an abnormal ‘founding’ cell. Possible factors initiating this change include intrinsic myometrial abnormalities, elevated oestrogen receptors in the myometrium, hormonal changes and ischaemic injury (Parker 2007).
It has long been known that fibroid growth is dependent on both oestrogen and progesterone. Fibroids only occur after the menarche, and they normally show a reduction in size after the menopause. They display reversible shrinkage after treatment with gonadotrophin-releasing hormone (GnRH) agonists. Although circulating serum oestradiol and progesterone levels are not altered in women with fibroids, fibroids have increased local oestrogen levels relative to the surrounding normal myometrium, partly due to increased fibroid expression of the aromatase enzyme (Parker 2007). They also have increased oestrogen receptor levels and increased responsiveness to oestrogen compared with normal myometrial cells. As for progesterone, the receptor levels have been shown to be increased in fibroids relative to the myometrium (Brandon et al 1993), and to upregulate the levels of some proteins which influence cell proliferation, such as the antiapoptotic protein Bcl-2 (Matsuo et al 1999). During the secretory phase of the menstrual cycle, when serum progesterone levels are at their peak, the highest mitotic activity is seen in fibroids. Similarly, high mitotic activity is seen during pregnancy. Treatment with the antiprogestin, mifepristone, has been shown to cause a marked decrease in fibroid tumour volume.
A large number of growth factors are produced locally in uterine fibroids and the myometrium. Some of them are overexpressed in fibroids, partially due to an exaggerated response to oestrogen. Some of the growth factors that have been found to have a potential role in the promotion of fibroid growth include: transforming growth factor-β, epidermal growth factor, basic fibroblast factor, insulin-like growth factor, platelet-derived growth factor and vascular endothelial growth factor (VEGF). The differential effects of these growth factors on cell proliferation, angiogenesis, extracellular matrix production and apoptosis are due to a variety of mechanisms including altered receptor levels and signalling pathways. For a detailed review, see Fleischer et al (2008).
Epidemiology
Age is the most common risk factor for uterine fibroids (Marshall et al 1997), although many of the studies examining incidence are cross-sectional or retrospective, rather than prospective and longitudinal. Race has been identified as an epidemiological factor in fibroid development. Black women are three to five times more likely to have uterine fibroids than White, Asian or Hispanic women (Marshall et al 1997). In addition, the fibroids in Black women are larger, more numerous, more symptomatic and diagnosed at an earlier age.
There is evidence of hereditary factors influencing fibroid development, despite no specific gene being identified. The development of fibroids clusters in families, and ‘familial’ fibroids appear to be more likely to cause symptoms than ‘non-familial’ fibroids (Okolo 2008). Twin studies show an increased incidence of fibroids in monozygotic twin pairs compared with dizygotic twin pairs.
Uterine fibroids are more common in nulliparous women, presumably due to the increased number of cycles over their premenopausal lifespan compared with parous women. Increasing parity decreases the incidence and number of fibroids. It is a myth that fibroids increase in size during pregnancy. In pregnancy, fibroids initially increase in size in the first trimester, but then decrease in size for the remaining two trimesters, with an overall volume that is either reduced or unchanged throughout the pregnancy (Hammoud et al 2006). Obesity, diabetes mellitus, polycystic ovary syndrome and hypertension all appear to be associated with an increased risk of uterine fibroids. In these conditions, a combination of insulin resistance, increased androgen levels and increased peripheral conversion of circulating androgens to oestrogen may alter the ovarian steroid hormonal stimulus to the fibroids. Smoking decreases the risk of uterine fibroids, presumably through a reduction in oestrogen levels.
The effects of exogenous sex steroid hormones on fibroid growth are particularly important as they comprise some of the most common treatments for menorrhagia, itself the most common symptom of uterine fibroids. Use of the combined oral contraceptive pill (COCP) has been associated with fibroid growth in some earlier studies (particularly older pill formulations with a higher oestrogen dosage), although the majority show no association or even a reduced risk of developing fibroids with COCP use. Therefore, fibroids are not a contraindication to the use of the COCP to treat menorrhagia. Both systemic and local administration of progestins to the uterus have been shown to be safe with fibroids. Depot medroxyprogesterone acetate injections reduce the risk of fibroids, and the levonorgestrel-containing intrauterine system (LNG-IUS; Mirena) reduces the overall volume of fibroid uteri. The LNG-IUS is also an effective treatment for menorrhagia associated with fibroids, although it is less effective than for dysfunctional uterine bleeding. In some patients, it may be difficult to insert the LNG-IUS due to cavity distortion by the fibroids. The effect of hormone therapy in postmenopausal women on fibroids has been reviewed by Ang et al (2001). They found that use of hormone therapy in women with fibroids caused continued fibroid growth, especially in the first 6 months of therapy, or at least a failure in normal postmenopausal regression. However, this did not correspond to an increase in symptoms.
Classification and Pathophysiology
Macroscopic appearance
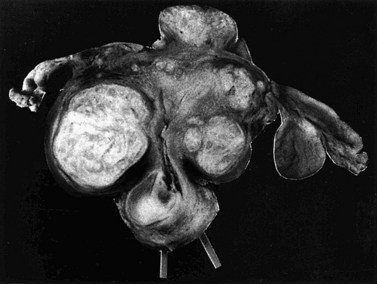
Fibroids have a characteristically firm fibrous texture, from which the clinical term ‘fibroid’ is presumably derived. They frequently become calcified; rarely, they become ossified so that they are rock hard. Fibroids are round or oval-shaped tumours with a characteristic white whorled appearance on cross-section. They may be single but are more commonly multiple, present in varying sizes and in different sites (Figure 32.1). Tiny ‘seedling’ fibroids are commonly seen in association with larger tumours, possibly accounting for the high recurrence rate after surgical removal at myomectomy. Surgical removal of over 120 individual fibroids from a single patient has been recorded in the literature!
Clinical classification
Subserosal fibroids
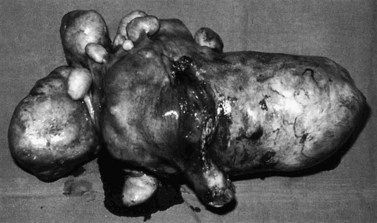
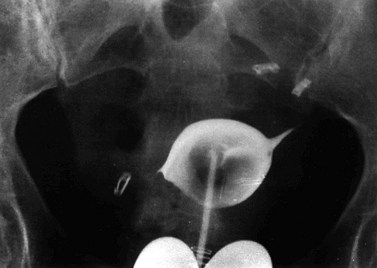
Subserosal fibroids project outwards from the uterine surface, covered with peritoneum, and may reach a very large size. Greater than 50% of the fibroid mass must project beyond the myometrium for the fibroid to be classified as subserosal. Many subserosal fibroids are pedunculated (Figure 32.2), making torsion a potential, although rare, complication. Sessile subserosal fibroids projecting from the fundal region may adhere to omentum or bowel, particularly if there has been coincidental inflammatory disease. Fibroids rarely become attached to the omentum; if they develop an alternative blood supply, they may become separated from the uterus, forming a so-called ‘parasitic’ fibroid. Subserosal fibroids arising from the lateral uterine wall may lie between the layers of the broad ligament where large tumours may displace the ureters, or bulge between the layers of the sigmoid mesocolon. Broad ligament fibroids arising from the lateral uterine wall differ from true broad ligament fibroids, which have no attachment to the uterus but have their origin in smooth muscle fibres within the broad ligament; for example, from the round ligament, ovarian ligament or perivascular connective tissue.
Submucosal fibroids
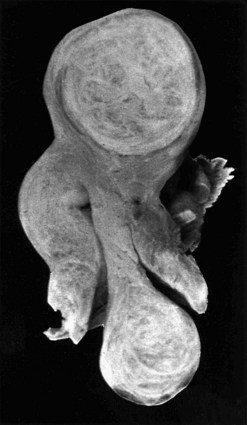
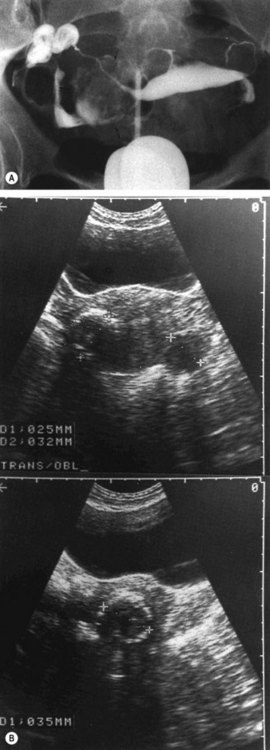
Submucosal fibroids are less common, comprising approximately 5% of all leiomyomata. By definition, more than 50% of the fibroid mass projects into the uterine cavity and is covered by endometrium. Submucosal fibroids may cause abnormal uterine bleeding and infertility. Uterine enlargement is not usually evident unless other fibroids are also present. Submucous fibroids are regarded as suitable for hysteroscopic resection. Pedunculated submucous fibroids on a long stalk may prolapse through the cervix (Figure 32.3), where they may cause intermenstrual bleeding or become ulcerated and infected.
Vascular supply
Perfusion studies with injections of 133Xe radiocontrast and gadolinium-enhancement contrast material during magnetic resonance imaging (MRI) show reduced blood flow through fibroids compared with myometrium, but increased blood flow in a compressed rim around the fibroid. Immunohistochemical studies of the microvascular density of fibroids demonstrate reduced vessel area staining in fibroids compared with the myometrium (Casey et al 2000), and with the difference increasing after the menopause (Weston et al 2005); this is probably due to relatively faster collapse of the myometrial cellular and extracellular matrix volume relative to that of the fibroid. Electron microscopy of corrosion vessel casts of fibroids performed by Walocha et al (2003) shed even greater insight into the vasculature of fibroids. A single larger artery usually provides the major blood supply to the fibroid, sending small nutrient arteries to penetrate the capsule. The vasculature of fibroids is reduced compared with the surrounding myometrium, but is most markedly reduced in smaller fibroids, with the only area of greater vascularity than the myometrium being the compressed rim of perifibroid tissue around the periphery of larger fibroids. Walocha et al (2003) proposed an initial regression of the vasculature within smaller fibroids, followed by an intense proangiogenic response, whereby the vascular rim around the fibroid acts as the source for neovascularization of the growing fibroid.
The relative avascularity of the fibroid core appears to be at odds with the finding of increased VEGF in fibroids compared with the myometrium. However, the VEGF within the interior of the fibroid may be trapped in a biologically inactive fashion within the expanded extracellular matrix of the fibroid. A gene expression microarray experiment performed by Weston et al (2003) specifically targeting angiogenesis-related genes found a reduced level of two angiogenesis promoters (CTGF and CYR61) and increased expression of collagen IVa 2; a precursor for the angiogenesis inhibitor, canstatin. This triad of gene expression differences may, in part, explain the relatively avascular fibroid tissue.
Microscopic appearance
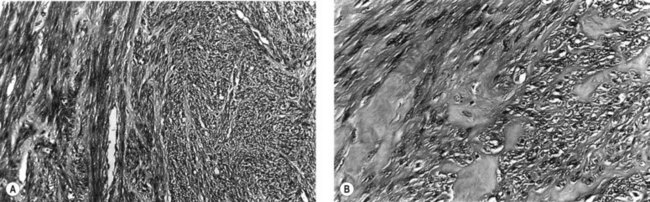
Microscopically, fibroids are composed of smooth muscle cell bundles, also arranged in whorl-like patterns (Figure 32.4A), admixed with a variable amount of connective tissue, although the latter rarely predominates. The relatively poor blood supply to individual fibroids may result in degenerative changes, particularly within large tumours. Hyaline degeneration (Figure 32.4B) is the most common, resulting in a smoother and more homogeneous consistency, which may become cystic if liquefaction occurs. Much more rarely, fatty change may develop. This is distinct from the true lipoma of the uterus, which is extremely uncommon. Calcification (Figure 32.5) is a later consequence of degeneration secondary to circulatory impairment. It characteristically occurs after the menopause, although it may occur earlier in subserosal fibroids with narrow pedicles.
Crow et al (1995) examined the microscopic appearance of uterine fibroids after treatment with GnRH agonists. They found considerable variation between fibroids after a course of treatment and subsequent myomectomy or hysterectomy. Decreases in the extracellular matrix, in particular, cause extreme crowding of tumour nuclei, resulting in a densely cellular appearance of such fibroids on microscopic examination. For the inexperienced pathologist, this will be worrying in terms of possible malignancy. There should not, however, be mitotic activity, cytological atypia or coagulative necrosis.
Clinical Presentation
Abnormal bleeding/menorrhagia
The proportion of uterine fibroids causing abnormal uterine bleeding — menorrhagia being the most common abnormal pattern observed — is uncertain but is usually quoted as 30–50%. The exact mechanism by which fibroids cause menorrhagia is unknown. The many theories include: increase in endometrial surface area (Figure 32.6), increased vascularity of the uterus, vascular congestion due to fibroid compression of the myometrial venous drainage, abnormal uterine contractility, dysregulation of growth factors causing disordered angiogenesis of the uterine vasculature, and ulceration of the endometrial layer overlying submucous fibroids.
As fibroids are so common, and so often asymptomatic, menorrhagia in a woman with uterine fibroids should not automatically be ascribed to the fibroids themselves. Other possible aetiologies for menorrhagia should always be considered and excluded, including coagulopathies such as von Willebrand’s disease (Munro and Lukes 2005). In a study of self-reported bleeding patterns by 878 women (aged 35–49 years) screened for the presence and size of myomas, Wegienka et al (2003) reported findings on the association between fibroids and bleeding pattern. Of women with fibroids, 46% reported ‘gushing’ episodes during their periods, compared with 28% without fibroids. Gushing episodes and length of periods were related to the size of the myomas, but not to their number or location. The worst bleeding was found with fibroids over 5 cm in diameter, with women using three more pads or tampons on heavy bleeding days than women with smaller fibroids.
Pelvic pain and pressure symptoms
Fibroids do not usually give rise to pain. In a cohort study of 635 non-care-seeking women, symptoms of dyspareunia, dysmenorrhoea and non-cyclic pelvic pain were examined for their association with the presence and size of uterine fibroids (Lippman et al 2003). The 96 women with fibroids had only a slight increase in non-cyclic pain or dyspareunia, but no increase in dysmenorrhoea, and no relationship was found between the size or total volume of fibroids and pain symptoms. Where fibroids are associated with pain or abdominal discomfort, it is usually described as ‘pressure’ or a ‘dragging’ sensation. It is surprising that even in the presence of multiple fibroids, menstruation may be painless.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree