Andrea Mariani
John R. Lurain
Endometrial carcinoma is the most common malignancy of the female genital tract, accounting for almost one-half of all gynecologic cancers in the United States. In 2011, an estimated 46,470 new cases and 8,120 cancer-related deaths are anticipated. Endometrial carcinoma is the fourth most common cancer, ranking behind breast, lung, and colorectal cancers, and the eighth leading cause of death from malignancy in women. Overall, about 2% to 3% of women develop endometrial cancer during their lifetimes (1). Certain factors are increasing awareness of and emphasis on diagnosis and treatment of endometrial cancer. These factors include the declining incidence of cervical cancer-related deaths in the United States, prolonged life expectancy, postmenopausal use of hormone therapy, and earlier diagnosis. The availability of easily applied diagnostic tools and a clearer understanding of premalignant lesions of the endometrium led to an increase in the number of women diagnosed with endometrial cancer. Although endometrial carcinoma usually presents as early-stage disease and often is managed without radical surgery or radiotherapy, deaths from endometrial carcinoma now exceed those from cervical carcinoma in the United States. Endometrial cancer is a disease that occurs primarily in postmenopausal women and is increasingly virulent with advancing age. The definite role of estrogen in the development of most endometrial cancers is established. Any factor that increases exposure to unopposed estrogen increases the risk for endometrial cancer.
The histopathology, spread patterns, and clinicopathologic factors that affect the prognosis of endometrial cancers have become better defined. Management of endometrial cancer evolved from a program of preoperative intrauterine or external pelvic radiation followed by hysterectomy based on clinical staging, to an individualized approach using hysterectomy as primary therapy and employing additional postoperative treatment depending on surgical and pathologic findings. Further analysis and investigation are needed to determine whether this initial operative approach to treatment and staging, followed by targeted postoperative therapy, will translate into improved survival rates and lower morbidity.
Table 35.1 Risk Factors for Endometrial Cancer
| Characteristic | Relative Risk |
| Nulliparity | 2–3 |
| Late menopause | 2.4 |
| Obesity | |
| 21–50 lb overweight | 3 |
| >50 lb overweight | 10 |
| Diabetes mellitus | 2.8 |
| Unopposed estrogen therapy | 4–8 |
| Tamoxifen therapy | 2–3 |
| Atypical endometrial hyperplasia | 8–29 |
| Lynch II syndrome | 20 |
Epidemiology and Risk Factors
There appear to be two pathogenetic types of endometrial cancer (2). Type I, accounting for about 75% to 85% of cases, occurs in younger, perimenopausal women with a history of exposure to unopposed estrogen, either endogenous or exogenous. In these women, tumors begin as hyperplastic endometrium and progress to carcinoma. These “estrogen-dependent” tumors tend to be better differentiated and have a more favorable prognosis than tumors that are not associated with hyperestrogenism. Type II endometrial carcinoma occurs in women without estrogenic stimulation of the endometrium. These spontaneously occurring cancers are not associated pathologically with endometrial hyperplasia, but may arise in a background of atrophic endometrium. They are less differentiated and associated with a poorer prognosis than estrogen-dependent tumors. These “estrogen-independent” tumors tend to occur in older, postmenopausal, thin women and are present disproportionately in AfricanAmerican and Asian women. Over the past decade, molecular genetic studies showed that these two tumor types evolve via distinct pathogenetic pathways (3) (see Type I and II Endometrial Carcinoma: Molecular Aberrations, below).
Several risk factors for the development of endometrial cancer are identified (4–9) (Table 35.1). Most of these risk factors are related to prolonged, unopposed estrogen stimulation of the endometrium. Nulliparous women have twoto threetimes the risk of parous women. Infertility and a history of irregular menses as a result of anovulatory cycles (prolonged exposure to estrogen without sufficient progesterone) increase risk. Natural menopause occurring after age 52 years increases the risk for endometrial cancer 2.4-fold compared with women who experienced menopause before 49 years of age, probably as a result of prolonged exposure of the uterus to progesterone-deficient menstrual cycles. The risk of endometrial cancer is increased 3 times in women who are 21 to 50 pounds overweight and 10 times in those more than 50 pounds overweight (resulting from excess estrone as a result of peripheral conversion of adrenallyderived androstenedione by aromatization in fat). The obesity epidemic in Western countries, together with growing rates of insulin resistance and “metabolic syndrome,” can be expected to increase the incidence of endometrial cancer in coming years.
Other factors leading to long-term estrogen exposure, such as polycystic ovary syndrome and functioning ovarian tumors, also are associated with an increased risk for endometrial cancer. Menopausal estrogen therapy without progestins increases the risk of endometrial cancer fourto eighttimes. This risk increases with higher doses and with more prolonged use and can be reduced to essentially baseline levels by the addition of progestin (8). The use of the antiestrogen tamoxifen for treatment of breast cancer is associated with a two- to threefold increased risk for the development of endometrial cancer, although this finding is confounded by the apparent greater risk of endometrial cancer in women who have breast cancer, with or without treatment with tamoxifen (9,10). Diabetes mellitus increases a women’s risk for endometrial cancer by 1.3 to 2.8 times. Women with Lynch II syndrome (previously referred to as hereditary nonpolyposis colorectal cancer syndrome, or HNPCC), a cancer susceptibility syndrome with germline mutations in mismatch repair genes MLH1, MSH2, and MSH6, have a 40% to 60% lifetime risk for endometrial and colon cancer (11). Other medical conditions, such as hypertension and hypothyroidism, are associated with endometrial cancer, but a causal relationship is not confirmed.
Table 35.2 Classification of Endometrial Hyperplasias
| Type of Hyperplasia | Progression to Cancer (%) |
| Simple (cystic without atypia) | 1 |
| Complex (adenomatous without atypia) | 3 |
| Atypical | |
| Simple (cystic with atypia) | 8 |
| Complex (adenomatous with atypia) | 29 |
| From Kurman RJ, Kaminski PF, Norris HJ. The behavior of endometrial hyperplasia: a long term study of “untreated” hyperplasia in 170 patients. Cancer 1985;56:403–412, with permission. | |
Endometrial Hyperplasia
Endometrial hyperplasia represents a spectrum of morphologic and biologic alterations of the endometrial glands and stroma, ranging from an exaggerated physiologic state to carcinoma in situ. Clinically significant hyperplasias usually evolve within a background of proliferative endometrium as a result of protracted estrogen stimulation in the absence of progestin influence. Endometrial hyperplasias are important clinically because they may cause abnormal bleeding, be associated with estrogen-producing ovarian tumors, result from hormonal therapy, and precede or occur simultaneously with endometrial cancer.
The classification scheme endorsed by the International Society of Gynecological Pathologists is based on architectural and cytologic features and long-term studies that reflect the natural history of the lesions (12) (Table 35.2). Architecturally, hyperplasias are either simple or complex; the major differing features are complexity and crowding of the glandular elements. Simple hyperplasia is characterized by dilated or cystic glands with round to slightly irregular shapes, an increased glandular-to-stromal ratio without glandular crowding, and no cytologic atypia. Complex hyperplasia has architecturally complex (budding and infolding) crowded glands, with less intervening stroma without atypia. Atypical hyperplasia refers to cytologic atypia and can be categorized as simple or complex, depending on the corresponding glandular architecture. Criteria for cytologic atypia include large nuclei of variable size and shape that have lost polarity, increased nuclear-to-cytoplasmic ratios, prominent nucleoli, and irregularly clumped chromatin with parachromatin clearing (Fig. 35.1).
Figure 35.1 Atypical hyperplasia (complex hyperplasia with severe nuclear atypia) of endometrium. A: The proliferative endometrial glands reveal considerable crowding and papillary infoldings. The endometrial stroma, although markedly diminished, can still be recognized between the glands. B: Higher magnification demonstrates disorderly nuclear arrangement and nuclear enlargement and irregularity. Some contain small nucleoli. (Provided by Gordana Stevanovic, MD, and Jianyu Rao, MD, Department of Pathology, UCLA.)
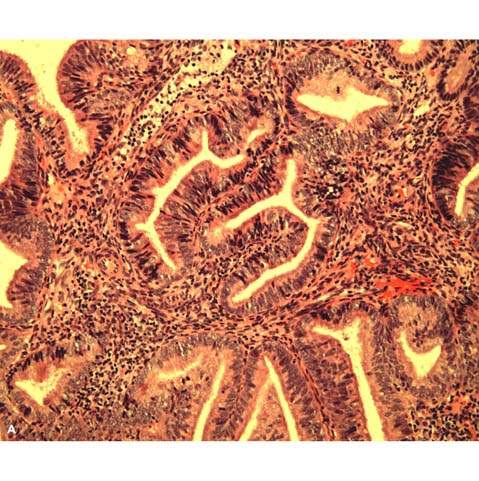
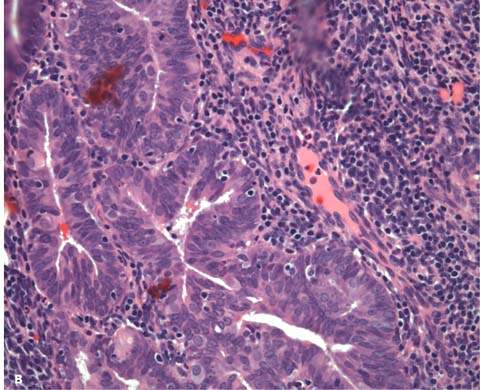
The risk of endometrial hyperplasia progressing to carcinoma is related to the presence and severity of cytologic atypia. Kurman et al. retrospectively studied endometrial curettings from 170 patients with untreated endometrial hyperplasia followed a mean of 13.4 years (13). They found that progression to carcinoma occurred in 1% of patients with simple hyperplasia, 3% of patients with complex hyperplasia, 8% of patients with atypical simple hyperplasia, and 29% of patients with atypical complex hyperplasia. Most of the hyperplasias seemed to remain stable (18%) or regress (74%). The premalignant potential of hyperplasia is influenced by age, underlying ovarian disease, endocrinopathy, obesity, and exogenous hormone exposure (14,15).
As many as 25% to 43% of patients with atypical hyperplasia detected in an endometrial biopsy or curettage specimen will have an associated, usually well-differentiated, endometrial carcinoma detected during hysterectomy (16). Marked cytologic atypia, a high mitotic rate, and marked cellular stratification are features of atypical endometrial hyperplasia most often associated with the finding of an undiagnosed carcinoma at hysterectomy.
Fertility Sparing Treatment of Endometrial Hyperplasia and Cancer
Younger patients with endometrial cancer tend to have disorders such as polycystic ovarian syndrome, chronic anovulation, and infertility, indicative of exposure to intrinsic estrogen excess (17). Lesions in this age group are usually welldifferentiated and of endometrioid subtype with the potential to regress with progestational therapy (18). Althoughstandard treatment for all endometrial cancer is hysterectomy and staging, nonsurgical treatment with hormonal therapy may be an option for appropriately selected women desiring to preserve fertility. Surrogate staging techniques, such as magnetic resonance imaging (MRI), may be employed to evaluate the depth of myometrial invasion or identify extrauterine disease (19,20). The sensitivity of MRI to evaluate these factors is limited and has the potential for underdiagnosis (21).
High regression rates for both endometrial cancer and atypical hyperplasia following treatment with progestin therapy are extensively documented (18,22–28). However, relatively small cohorts of patients and reports of hormone failure suggest caution when counseling patients for conservative management (27,29). In a 2004 meta-analysis, Ramirez et al. reported a comprehensive review of hormonal treatment of grade 1endometrial cancer, including 27 articles with a combined total of 81 patients. A variety of progestational agents were utilized with an overall response rate of 76% (62/81) and the median time to regression was 12 weeks (30). The recurrence rate was 24% among responders; nearly all recurrences occurred within 1year of diagnosis. Only 1month of progestational treatment was required to achieve a response in the 76% of patients without recurrence. Twenty patients achieved pregnancy following treatment. It is important to note that 24% (19/81) of the original cohort never responded to treatment, and only 68% had any documented follow-up endometrial sampling. Progestational therapy can successfully treat disease while preserving fertility for patients with atypical hyperplasia and well-differentiated presumed stage I endometrial cancer. Appropriate patient selection and exclusion criteria remain undefined. Patients must be counseled that failure to identify recurrence or extension of disease during progestational treatment may lead to a delay in definitive surgery and ultimately a compromised prognosis (27).
Continuous progestin therapy with megestrol acetate (40–160 mg per day) is probably the most reliable treatment for reversing complex or atypical hyperplasia. No clear consensus exists for an optimal follow-up interval. Therapy should be continued for at least 2 to 3 months, and endometrial biopsy should be performed 3 to 4 weeks after completion of therapy to assess response. Periodic endometrial biopsy or transvaginal ultrasonography is advisable in patients being monitored after progestin therapy for atypical hyperplasia because of the presence of undiagnosed cancer in 25% of cases, the 29% progression rate to cancer, and the high recurrence rate after treatment with progestins. In this setting the use of progesterone should be considered a temporary, rather than long-term, treatment. For women with atypical complex hyperplasia who no longer desire fertility, hysterectomy is recommended.
Endometrial Cancer Screening in the General Population
Screening for endometrial cancer should not be undertaken because of the lack of an appropriate, cost-effective, and acceptable test that reduces mortality (31–33). Routine Papanicolaou (Pap) testing is inadequate, and endometrial cytologic assessment is too insensitive and nonspecific to be useful in screening for endometrial cancer, even in a high-risk population. A progesterone challenge test reveals whether the endometrium is primed by estrogen, but it does not identify abnormal endometrial pathology. Transvaginal ultrasonographic examination of the uterus and endometrial biopsy are too expensive to be employed as screening tests.
Although many risk factors for endometrial cancer have been identified, screening of high-risk individuals using current technologies could, at best, detect only one-half of all cases of endometrial cancer. Furthermore, no controlled trials were carried out to evaluate the effectiveness of screening for endometrial cancer. Screening for endometrial cancer or its precursors may be justified for certain high-risk women, such as those receiving postmenopausal estrogen therapy without progestins and members of families with hereditary nonpolyposis colorectal cancer (34). Women taking tamoxifen receive no benefit from routine screening with transvaginal ultrasonography or endometrial biopsy (35,36).
Most patients who have endometrial cancer present with abnormal perimenopausal or postmenopausal uterine bleeding early in the development of the disease, when the tumor is still confined to the uterus. Application of an appropriate and accurate diagnostic test in this situation usually results in early diagnosis, timely treatment, and a high cure rate. It is important to recognize that the workup of abnormal uterine bleeding should include endometrial biopsy even in premenopausal patients as 5% are in women under the age of 40.
Surveillance and Prevention in Patients at High Risk
Most endometrial carcinomas are sporadic, but about 10% of cases have a hereditary basis (37–41). Two genetic models were described in the development of familial endometrial cancer: HNPCC or Lynch II syndrome and a predisposition for endometrial cancer alone; both are inherited in an autosomal dominant fashion (42). The majority of studies focused on the increased incidence of endometrial cancer associated with Lynch II syndrome, a highly penetrant disorder (80% to 85%) (43). HNPCC or Lynch II syndrome is caused by an inherited mutation in one of the following mismatch repair genes: hMSH2, hMLH1, PMS1, PMS2, or hMSH6 (44–47). The disorder is characterized by early age (average age younger than 45 years) at onset of neoplastic lesions in a variety of tissues, including the colon, uterus, stomach, ureters, ovaries, and skin (43,48,49). The lifetime risk of endometrial cancer in women with Lynch II syndrome is 32% to 60% and the lifetime risk of ovarian cancer is 10% to 12% (50,51). Interestingly, colorectal cancer is less prevalent in women with HNPCC or Lynch II syndrome than in men, whose risk approaches 100%. In a study of 1,763 patients from 50 HNPCC or Lynch II syndrome families in the Finnish Cancer Registry, the cumulative incidence of colorectal cancer in women was 54% by age 70, while the cumulative incidence of endometrial cancer was 60% (11). Although these data appear to support the use of endometrial cancer surveillance strategies for women with Lynch syndrome, a specific algorithm is not defined (50,52). No effective screening method exists for patients at increased risk for ovarian cancer.
In 2006, a European workshop of 21 experts in the treatment of hereditary gastrointestinal cancers from ninecountries (the Mallorca group) recommended the following endometrial cancer surveillance strategy for patients with HNPCC or Lynch II syndrome: annual pelvic examination, transvaginal ultrasound, and endometrial biopsy beginning at 30 to 35 years of age (53). These recommendations are by expert opinion only, and it is unknown whether these interventions are cost-effective or will impact mortality from endometrial or ovarian cancer in patients with Lynch II syndrome. An attractive alternative to early detection is prophylactic surgery after completion of childbearing (54,55). In 2006 a multi-institutional, matched case-control study found that prophylactic hysterectomy withbilateral salpingo-oophorectomy is an effective primary prevention strategy in women with Lynch II syndrome (51). No woman with hysterectomy and bilateral salpingo-oophorectomy developed endometrial, ovarian, or primary peritoneal carcinoma during the period of follow-up. In contrast, endometrial cancer developed in 33% and ovarian cancer in 5% of women who did not undergo prophylactic surgery (51).
There are rare reports of pedigrees in which family members are affected by endometrial cancer alone, and genetic studies have not found a germline mutation associated with site-specific endometrial cancer (42,56,57). A population-based study of endometrial cancer and familial risk in younger women (Cancer and Steroid Hormone, or CASH, Study Group) reported that a history of endometrial cancer in a first-degree relative increased the risk of endometrial cancer by nearly threefold (odds ratio of 2.8; 95% confidence interval [CI], 1.9–4.2) (58). A significant association was found with colorectal cancers, with an observed odds ratio of 1.9 (95% CI, 1.1–3.3). The presence of Lynch II syndrome families within the cohort may explain the latter association, but a family history of endometrial caner was an independent risk factor for endometrial cancer, after adjusting for age, obesity, and number of relatives (58).
Endometrial cancer and breast cancer share some of the same reproductive and hormonal risk factors such as nulliparity and exposure to unopposed estrogen (4,8,59–63). However, the familial association between breast and endometrial cancer is still uncertain and studies report conflicting results (63–67). For example, in the past it was thought that patients with BRCA mutations were at elevated risk for endometrial cancer, in addition to breast and ovarian cancer. A study suggests that this increase in risk is seen only in those patients with a personal history of breast cancer who are taking tamoxifen (68).
Endometrial Cancer
Clinical Features
Symptoms
Endometrial carcinoma most often occurs in women in the sixth and seventh decades of life, at an average age of 60 years; 75% of cases occur in women older than 50 years of age. About 90% of women with endometrial carcinoma have vaginal bleeding or discharge as their only presenting symptom. Most women recognize the importance of this symptom and seek medical consultation within 3 months. Some women experience pelvic pressure or discomfort indicative of uterine enlargement or extrauterine disease spread. Bleeding may not have occurred because of cervical stenosis, especially in older patients, and may be associated with hematometra or pyometra, causing a purulent vaginal discharge. This finding is often associated with a poor prognosis (69). Less than 5% of women diagnosed with endometrial cancer are asymptomatic. In the absence of symptoms, endometrial cancer usually is detected as the result of investigation of abnormal Pap test results, discovery of cancer in a uterus removed for some other reason, or evaluation of an abnormal finding on a pelvic ultrasonography examination or computed tomography (CT) scan obtained for an unrelated reason. Women who are found to have malignant cells on Pap test are more likely to have a more advanced stage of disease (70).
Abnormal perimenopausal and postmenopausal bleeding should always be taken seriously and be properly investigated, no matter how minimal or nonpersistent. Causes may be nongenital, genital extrauterine, or uterine (71). Nongenital tract sites should be considered based on the history or examination, including testing for blood in the urine and stool.
Table 35.3 Causes of Postmenopausal Uterine Bleeding
| Cause of Bleeding | Percentage |
| Endometrial atrophy | 60–80 |
| Estrogen replacement therapy | 15–25 |
| Endometrial polyps | 2–12 |
| Endometrial hyperplasia | 5–10 |
| Endometrial cancer | 10 |
Invasive tumors of the cervix, vagina, and vulva are usually evident on examination, and any tumors discovered should be biopsied. Traumatic bleeding from an atrophic vagina may account for up to 15% of all causes of postmenopausal vaginal bleeding. This diagnosis can be considered if inspection reveals a thin, friable vaginal wall, but the possibility of a uterine source of bleeding must first be eliminated.
Possible uterine causes of perimenopausal or postmenopausal bleeding include endometrial atrophy, endometrial polyps, estrogen therapy, hyperplasia, and cancer or sarcoma (72–75) (Table 35.3). Uterine leiomyomas should never be accepted as a cause of postmenopausal bleeding. Endometrial atrophy is the most common endometrial finding in women with postmenopausal bleeding, accounting for 60% to 80% of such bleeding. Women with endometrial atrophy usually were menopausal for about 10 years. Endometrial biopsy often yields insufficient tissue or only blood and mucus, and usually bleeding ceases after biopsy. Endometrial polyps account for 2% to 12% of postmenopausal bleeding. Polyps are often difficult to identify with office endometrial biopsy or curettage. Hysteroscopy, transvaginal ultrasonography, or both may be useful adjuncts in identifying endometrial polyps. Unrecognized and untreated polyps may be a source of continued or recurrent bleeding, leading eventually to unnecessary hysterectomy.
Estrogen therapy is an established risk factor for endometrial hyperplasia and cancer. The risk for endometrial cancer is fourto eighttimes greater in postmenopausal women receiving unopposed estrogen therapy, and the risk increases with time and higher estrogen doses. This risk can be decreased by the addition of a progestin to the estrogen, either cyclically or continuously. Endometrial biopsy should be performed as indicated to assess unscheduled bleeding or annually in women not taking a progestin. Endometrial hyperplasia occurs in 5% to 10% of patients with postmenopausal uterine bleeding. The sources of excess estrogen should be considered, including obesity, exogenous estrogen, or an estrogen-secreting ovarian tumor. Only about 10% of patients with postmenopausal bleeding have endometrial cancer.
Premenopausal women with endometrial cancer invariably have abnormal uterine bleeding, which is often characterized as menometrorrhagia or oligomenorrhea, or cyclical bleeding that continues past the usual age of menopause. The diagnosis of endometrial cancer must be considered in premenopausal women if abnormal bleeding is persistent or recurrent or if obesity or chronic anovulation is present.
Signs
Physical examination seldom reveals any evidence of endometrial carcinoma, although obesity and hypertension are commonly associated constitutional factors. Special attention should be given to the more common sites of metastasis. Peripheral lymph nodes and breasts should be assessed carefully. Abdominal examination is usually unremarkable, except in advanced cases in which ascites or hepatic or omental metastases may be palpable. On gynecologic examination, the vaginal introitus and suburethral area, and the entire vagina and cervix, should be carefully inspected and palpated. Bimanual rectovaginal examination should be performed specifically to evaluate the uterus for size and mobility, the adnexa for masses, the parametria for induration, and the cul-de-sac for nodularity.
Diagnosis
Office endometrial aspiration biopsy is the accepted first step in evaluating a patient with abnormal uterine bleeding or suspected endometrial pathology (76). The diagnostic accuracy of office-based endometrial biopsy is 90% to 98% when compared with subsequent findings at dilation and curettage (D&C) or hysterectomy (77–79).
The narrow plastic cannulas are relatively inexpensive, often can be used without a tenaculum, cause less uterine cramping (resulting in increased patient acceptance), and are successful in obtaining adequate tissue samples in more than 95% of cases. If cervical stenosis is encountered, a paracervical block can be performed, and the cervix can be dilated. Premedication with an antiprostaglandin agent can reduce uterine cramping. Complications following endometrial biopsy are exceedingly rare; uterine perforation occurs in only 1 to 2 cases per 1,000. Endocervical curettage may be performed at the time of endometrial biopsy if cervical pathology is suspected. A Pap test is an unreliable diagnostic test because only 30% to 50% of patients with endometrial cancer have abnormal Pap test results (80).
Hysteroscopy and D&C should be reserved for situations in which cervical stenosis or patient tolerance does not permit adequate evaluation by aspiration biopsy, bleeding recurs after a negative endometrial biopsy, or the specimen obtained is inadequate to explain the abnormal bleeding. Hysteroscopy is more accurate in identifying polyps and submucous myomas than endometrial biopsy or D&C alone (81–83).
Transvaginal ultrasonography may be a useful adjunct to endometrial biopsy for evaluating abnormal uterine bleeding and selecting patients for additional testing (84–87). Transvaginal ultrasonography, with or without endometrial fluid instillation (sonohysterography), may be helpful in distinguishing between patients with minimal endometrial tissue whose bleeding is related to perimenopausal anovulation or postmenopausal atrophy and patients with significant amounts of endometrial tissue or polyps who are in need of further evaluation. The finding of an endometrial thickness greater than 4 mm, a polypoid endometrial mass, or a collection of fluid within the uterus requires further evaluation. Although most studies agree that an endometrial thickness of 5 mm or less in a postmenopausal woman is consistent with atrophy, more data are needed before ultrasonography findings can be considered to eliminate the need for endometrial biopsy in a patient with symptoms (88).
Pathology
The histologic classification of carcinoma arising in the endometrium is shown in Table 35.4 (12,89).
Table 35.4 Classification of Endometrial Carcinomas
| Endometrioid adenocarcinoma |
| Variants |
| Villoglandular or papillary |
| Secretory |
| With squamous differentiation |
| Mucinous carcinoma |
| Papillary serous carcinoma |
| Clear cell carcinoma |
| Squamous carcinoma |
| Undifferentiated carcinoma |
| Mixed carcinoma |
Endometrioid Adenocarcinoma
The endometrioid type of adenocarcinoma accounts for about 80% of endometrial carcinomas. These tumors are composed of glands that resemble normal endometrial glands; they have columnar cells with basally oriented nuclei, little or no intracytoplasmic mucin, and smooth intraluminal surfaces (Fig. 35.2). As tumors become less differentiated, they contain more solid areas, less glandular formation, and more cytologic atypia. The well-differentiated lesions may be difficult to separate from atypical hyperplasia.
Criteria that indicate the presence of invasion and are used to diagnose carcinoma are desmoplastic stroma, back-to-back glands without intervening stoma, extensive papillary pattern, and squamous epithelial differentiation. These changes, with the exception of the infiltrating pattern with desmoplastic reaction, require an area of involvement equal to or exceeding one-half of a low-power microscopic field (LPF) (>1 LPF; 4.2 mm in diameter) (90,91).
The differentiation of a carcinoma, expressed as its grade, is determined by architectural growth pattern and nuclear features (Table 35.5). In the International Federation of Gynecology and Obstetrics (FIGO) grading system proposed in 1989, tumors are grouped into three grades: grade 1, 5% or less of the tumor shows a solid growth pattern; grade 2, 6% to 50% of the tumor shows a solid growth pattern; and grade 3, more than 50% of the tumor shows a solid growth pattern. The presence of notable nuclear atypia that is inappropriate for the architectural grade increases the tumor grade by one.
Adenocarcinomas with squamous differentiation are graded according to the nuclear grade of the glandular component. This FIGO system is applicable to all endometrioid carcinomas, including its variants, and to mucinous carcinomas. In serous and clear cell carcinomas, nuclear grading takes precedence; however, most investigators believe that these two carcinomas should always be considered high-grade lesions, making grading unnecessary.
About 15% to 25% of endometrioid carcinomas have areas of squamous differentiation (Fig. 35.3). In the past, tumors with benign-appearing squamous areas were called adenoacanthomas, and tumors with malignant-looking squamous elements were called adenosquamous carcinomas. It is recommended that the term endometrial carcinoma with squamous differentiation be used to replace these two designations because the degree of differentiation of the squamous component parallels that of the glandular component, and the behavior of the tumor is largely dependent on the grade of the glandular component (92,93).
Figure 35.2 Well-differentiated adenocarcinoma of endometrium. The glands and complex papillae are in direct contact with no intervening endometrial stroma, the so-called back-to-back pattern. (Provided by Gordana Stevanovic, MD, and Jianyu Rao, MD, Department of Pathology, UCLA.)
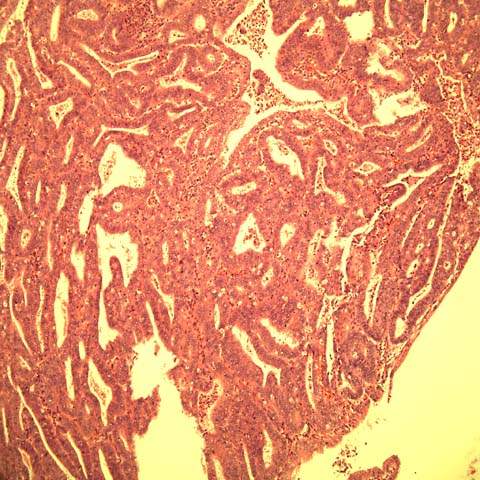
Table 35.5 FIGO Definition for Grading of Endometrial Carcinoma
| Histopathologic degree of differentiation: |
| G1 <5% nonsquamous or nonmorular growth pattern |
| G2 6%–50% nonsquamous or nonmorular growth pattern |
| G3 >50% nonsquamous or nonmorular growth pattern |
| Notes on pathologic grading: |
| Notable nuclear atypia, inappropriate for the architectural grade, raises a grade 1 (G1) |
| or grade 2 (G2) tumor by one grade. |
| In serous adenocarcinoma, clear cell adenocarcinoma, and squamous cell carcinoma, |
| nuclear grading takes precedence. |
| Adenocarcinomas with squamous differentiation are graded according to the nuclear grade |
| of the glandular component. |
| FIGO Committee on Gynecologic Oncology. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynecol Obst 2009;105:103–104. |
Figure 35.3 Adenocarcinoma with squamous differentiation of endometrium. This lesion is also classified as adenoacanthoma. Squamous cells with eosinophilic cytoplasm and distinct cell borders form solid clusters in the lumina of neoplastic glands. (Provided by Gordana Stevanovic, MD, and Jianyu Rao, MD, Department of Pathology, UCLA.)
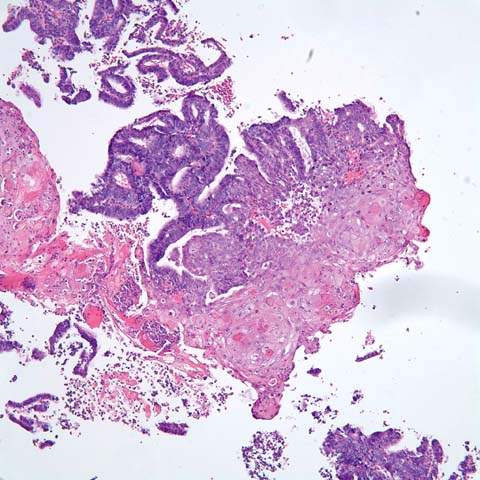
Avilloglandular configuration is present in about 2% of endometrioid carcinomas (94,95). In these tumors, the cells are arranged along fibrovascular stalks, giving a papillary appearance but maintaining the characteristics of endometrioid cells. The villoglandular variants of endometrioid carcinomas are always well-differentiated lesions that behave like the regular endometrioid carcinomas, and they should be distinguished from serous carcinomas. Secretory carcinoma is a rare variant of endometrioid carcinoma that accounts for about 1% of cases (96,97). It occurs mostly in women in their early postmenopausal years. The tumors are composed of well-differentiated glands with intracytoplasmic vacuoles similar to early secretory endometrium. These tumors behave as regular well-differentiated endometrioid carcinomas and have an excellent prognosis. Secretory carcinoma may be an endometrioid carcinoma that exhibits progestational changes, but a history of progestational therapy is rarely elicited. Secretory carcinoma must be differentiated from clear cell carcinoma because both tumors have predominately clear cells. These two tumors can be distinguished by their structure: secretory carcinomas have uniform glandular architecture, uniform cytology, and low nuclear grade, whereas clear cell carcinomas have more than one architectural pattern and a high nuclear grade.
Mucinous Carcinoma
About 5% of endometrial carcinomas have a predominant mucinous pattern in which more than one-half of the tumor is composed of cells with intracytoplasmic mucin (98,99). Most of these tumors have a well-differentiated glandular architecture; their behavior is similar to that of common endometrioid carcinomas, and the prognosis is good. It is important to recognize mucinous carcinoma of the endometrium as an entity and to differentiate it from endocervical adenocarcinoma. Features that favor a primary endometrial carcinoma are the merging of the tumor with areas of normal endometrial tissue, presence of foamy endometrial stromal cells, presence of squamous metaplasia, or presence of areas of typical endometrioid carcinoma. Positive perinuclear immunohistochemical staining with vimentin suggests an endometrial origin (100).
Serous Carcinoma
About 3% to 4% of endometrial carcinomas resemble serous carcinoma of the ovary and fallopian tube (101–104). Most often, these tumors are composed of fibrovascular stalks lined by highly atypical cells with tufted stratification (Fig. 35.4). Psammoma bodies frequently are observed.
Figure 35.4 Serous carcinoma of endometrium. Branching papillae are supported by delicate fibrovascular cores and lined with columnar cells with moderate nuclear atypism, multiple nucleoli, and mitotic figures. (Provided by Gordana Stevanovic, MD, and Jianyu Rao, MD, Department of Pathology, UCLA.)
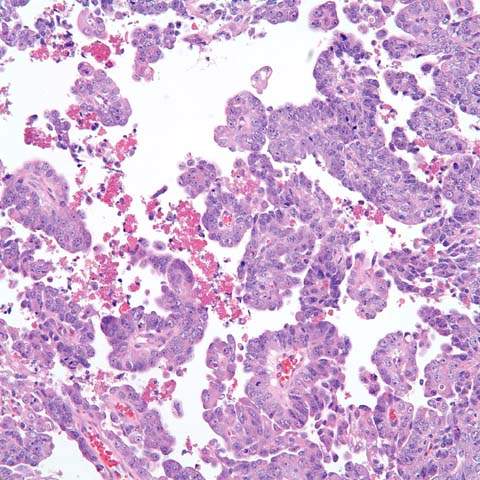
Serous carcinomas, also referred to as uterine papillary serous carcinomas, are considered high-risk lesions. The first description in 1982, noted that this entity usually occurred in elderly, hypoestrogenic women who presented with advanced-stage disease and accounted for up to one-half of deaths from endometrial carcinoma (101). Since then, several reports documented the aggressive nature and poor prognosis of serous carcinomas.
They are commonly admixed with other histologic patterns, but mixed tumors behave as aggressively as pure serous carcinomas. Even patients with a very small proportion of serous features (5%) remain at high risk of recurrence (105). Serous carcinomas are often associated with lymph–vascular space and deep myometrial invasion. The presence of lymph node metastases, positive peritoneal cytology, and intraperitoneal tumor does not necessarily correlate with increasing myometrial invasion (104). Even when these tumors appear to be confined to the endometrium or endometrial polyps without myometrial or vascular invasion, they behave more aggressively than endometrioid carcinomas and have a propensity to spread intra-abdominally, simulating the behavior of ovarian carcinoma. In one series, 37% of patients with serous carcinomas of the endometrium confined to a polyp demonstrated extrauterine disease when subjected to exploration and surgical staging (106).
A multi-institutional review of 206 patients with surgical stage I and II serous carcinomas demonstrated recurrence in 21% (105). Substage and treatment with platinum-based chemotherapy were associated with improved overall survival. Survival of surgically staged patients without myometrial invasion or extrauterine disease is between 89% and 100%, suggesting that observation may be appropriate in select patients, particularly in elderly patients with comorbidities (107). However, stage I patients, particularly those with myometrial invasion, remain at high risk of both peritoneal and vaginal recurrence. Therefore, platinum-based chemotherapy and vaginal brachytherapy should be considered in these patients (107–109).
Surgical treatment of advanced disease is no different fromthe endometrioid subtype, consisting of complete extirpation of visible disease (108). In one investigation from the Mayo Clinic, cytoreduction to microscopic residual was associated with a median overall survival of 51 versus 12 months for those patients with any residual (110). Postoperative treatment of advanced disease in the United States consists of chemotherapy and pelvicradiation, with or withoutpara-aortic radiation. The Gynecologic Oncology Group study GOG184 included serous carcinomas and randomized patients to carboplatin and paclitaxel versus cisplatin, doxorubicin (Adriamycin), and paclitaxel together with tumor volume–directed radiation (111). The former regimen demonstrated similar outcomes with less toxicity. Limited data suggest that delivering radiation “sandwiched” with chemotherapy improves progression-free and overall 3-year survival rates (112). Ongoing studies are evaluating the role of chemotherapy alone for these tumors, especially because of the high rate of peritoneal dissemination and recurrences. It remains unknown whether radiation improves survival in addition to chemotherapy alone. For elderly patients with multiple comorbidities who cannot tolerate multimodal therapy, chemotherapy alone.
Clear Cell Carcinoma
Clear cell carcinoma accounts for less than 5% of all endometrial carcinomas (96,113,114). Clear cell carcinoma usually has a mixed histologic pattern, including papillary, tubulocystic, glandular, and solid types. The cells have highly atypical nuclei and abundant clear or eosinophilic cytoplasm. Often, the cells have a hobnail configuration arranged in papillae with hyalinized stalks (Fig. 35.5).
Figure 35.5 Clear cell adenocarcinoma of the endometrium. Back-to-back glands lined by polygonal to columnar cells with distinct cell membrane, abundant granular to clear cytoplasm, and variably sized nuclei (including binucleated and multinucleated forms) with prominent nucleoli (magnification X400). (Provided by Gordana Stevanovic, MD, and Jianyu Rao, MD, Department of Pathology, UCLA.)
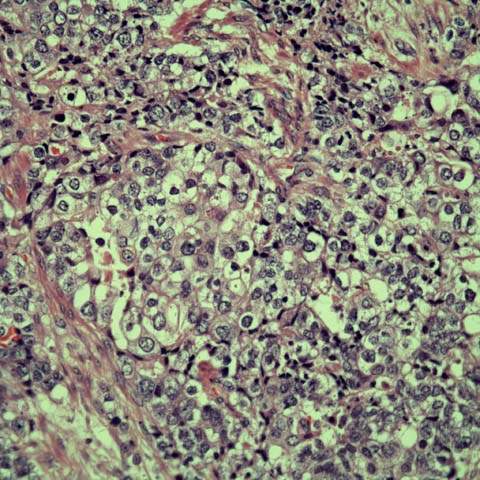
Clear cell carcinoma characteristically occurs in older women and like serous carcinoma is considered a poor prognosticator. Traditionally clear cell carcinoma was associated with very poor outcomes with overall survival rates varying from 33% to 64%. A multi-institutional review of 99 patients with uterine clear cell carcinoma documented only 1 recurrence (vaginal) in the 22 patients without extrauterine disease subjected to thorough surgical staging (115). Considering all 49 patients with stage I or II disease (regardless of the extent of staging), only 1 hematologic failure was noted. These data argue against the use of systemic therapy in patients with clear cell carcinoma limited to the pelvis, while the 10% vaginal cuff failure suggests that vaginal brachytherapy alone may be sufficient treatment. In contrast, others argued for systemic treatment of patients with stage I disease (116).
Complete surgical staging is important because 52% of patients with clinical stage I clear cell carcinoma have metastatic disease. Patients who undergo a complete cytoreduction appear to have improved progression-free and overall survivals compared to women left with residual disease following surgery (115). Postoperative therapy for patients with advanced disease is platinum-based (116).
Squamous Carcinoma
Squamous carcinoma of the endometrium is rare. Some tumors are pure, but most have a few glands. To establish primary origin within the endometrium, there must be no connection with or spread from cervical squamous epithelium. Squamous carcinoma often is associated with cervical stenosis, chronic inflammation, and pyometra at the time of diagnosis. This tumor has a poor prognosis, with an estimated 36% survival rate in patients with clinical stage I disease (117).
Simultaneous Tumors of the Endometrium and Ovary
Synchronous endometrial and ovarian cancers are the most frequent simultaneously occurring genital malignancies, with a reported incidence of 1.4% to 3.8% (118–122). Most commonly, both the ovarian and endometrial tumor are well-differentiated endometrioid adenocarcinomas of low stage, resulting in an excellent prognosis. Patients often are premenopausal and present with abnormal uterine bleeding. The ovarian cancer usually is discovered as an incidental finding and is diagnosed at an earlier stage because of the symptomatic endometrial tumor, leading to a more favorable outcome. Up to 29% of patients with endometrioid ovarian adenocarcinomas have associated endometrial cancer. If more poorly differentiated, nonendometrioid histologic subtypes are present or if the uterine and ovarian tumors are histologically dissimilar, the prognosis is less favorable. Immunohistochemical studies, flow cytometry, and assessment of molecular DNA patterns to detect loss of heterozygosity may be helpful in distinguishing between metastatic and independent tumors, but the differential diagnosis can usually be determined by conventional clinical and pathologic criteria.
Pretreatment Evaluation
After establishing the diagnosis of endometrial carcinoma, the next step is to evaluate the patient thoroughly to determine the best and safest approach to management of the disease. A complete history and physical examination areof utmost importance. Patients with endometrial carcinoma are often elderly and obese with a variety of medical problems, such as diabetes mellitus and hypertension, which complicate surgical management. Any abnormal symptoms, such as bladder or intestinal symptoms, should be evaluated.
On physical examination, attention should be directed to enlarged or suspicious lymph nodes, including the inguinal area, abdominal masses, and possible areas of cancer spread within the pelvis. Evidence of distant metastasis or locally advanced disease in the pelvis, such as gross cervical involvement or parametrial spread, may alter the treatment approach.
Chest radiography should be performed to exclude pulmonary metastasis and to evaluate the cardiorespiratory status of the patient. Other routine preoperative studies should include electrocardiography, complete blood and platelet counts, serum chemistries (including renal and liver function tests), and blood type and screen. Other preoperative or staging studies are neither required nor necessary for most patients with endometrial cancer. Studies such as cystoscopy, colonoscopy, intravenous pyelography, and barium enema are not indicated unless dictated by patient symptoms, physical findings, or other laboratory tests (123). CT scanning of the abdomen and pelvis may be considered in patients with type II uterine cancer to determine if minimally invasive surgery is appropriate. Stage IV disease is usually clinically evident based on patient symptomatology and clinical examination. Ultrasonography and MRI can be used to assess myometrial invasion preoperatively with a fairly high degree of accuracy (124). This information may be of use in planning the surgical procedure with regard to whether lymph node sampling should be undertaken.
Serum CA125, an antigenic determinant that is elevated in 80% of patients with advanced epithelial ovarian cancers, is elevated in most patients with advanced or metastatic endometrial cancer (125). In one study, 23 of 81 patients with apparently localized disease preoperatively had elevated CA125 levels. At surgery, 20 (87%) of these 23 patients with an elevated CA125 were found to have extrauterine disease, whereas only 1 of 58 patients with a normal CA125 had disease spread outside the uterus (126). Another study found that 78% of endometrial cancer patients with lymph node metastases had an elevated preoperative CA125 level (127). Preoperative measurement of serum CA125 may help determine the extent of surgical staging and, if elevated, may be useful as a tumor marker in assessing response to subsequent therapy (128,129).
Clinical Staging
Clinical staging, according to the 1971 FIGO system, should be performed only in patients who are deemed not to be surgical candidates because of their poor medical condition or the degree of disease spread (130). The current FIGO staging is surgical, as discussed below, which has supplanted the old clinical system. With improvements in preoperative and postoperative care, anesthesia administration, and surgical techniques, almost all patients are medically suitable for operative therapy. One study reported an operability rate of 87% in a series of 595 consecutive patients with clinical early-stage endometrial cancer (131). A small percentage of patients will not be candidates for surgical staging because of gross cervical involvement, parametrial spread, invasion of the bladder or rectum, or distant metastasis.
Surgical Staging
Widely accepted management of endometrial cancer consists of hysterectomy, removal of remaining adnexal structures, and appropriate surgical staging in patients considered at risk for extrauterine disease (132–134). Surgical staging was recommended for patients with endometrial cancer since 1988 (134). In spite of this general recommendation, the incorporation of a systematic pelvic and para-aortic lymphadenectomy in all patients is not universally accepted (135–137). This recommendation became more controversial after the publication of two large prospective randomized trials that failed to demonstrate improved outcomes for patients who underwent pelvic lymphadenectomy (138,139). These two studies show differences in their design: in the ASTEC trial all women with clinical stage I were included without exclusion criteria, whereas the Italian study excluded women with stage IA and IB grade 1 tumors, and nonendometrioid malignancies. In the Italian study, systematic nodal dissection was performed, as opposed to pelvic node sampling in the ASTEC trial (median number of lymph nodes harvested 30 vs. 12, respectively). The studies share characteristics that could lead to misinterpretation of their results. The percentage of nodal positivity is low in both studies (13% and 9%), suggesting that regardless of differences in exclusion criteria, low-risk cases were included in both studies, thus diluting possible (if any) therapeutic benefit of lymphadenectomy. Another important limitation is that nodal dissection was limited to the pelvis without any recommendation for para-aortic lymphadenectomy. It was demonstrated previously that radiotherapy limited to the pelvis does not improve survival (136). It is not surprising that pelvic lymphadenectomy alone has no therapeutic impact, considering that 67% of patients with nodal involvement have para-aortic lymph node metastases and 16% of patients with documented lymphatic dissemination have isolated para-aortic metastases (140). Neither study used the information derived from lymphadenectomy to target postoperative treatment (i.e., to spare patients with negative nodes from radiotherapy or to target postoperative treatment to the metastatic areas), thus eliminating one of the potential benefits of this surgical procedure.
Table 35.6 Carcinoma of the Endometrium (2008)
| Stage I∗ | Tumor confined to the corpus uteri |
| IA∗ | No or less than half myometrial invasion |
| IB∗ | Invasion equal to or more than half of the myometrium |
| Stage II∗ | Tumor invades cervical stroma, but does not extend beyond the uterus∗∗ |
| Stage III∗ | Local and/or regional spread of the tumor |
| IIIA∗ | Tumor invades the serosa of the corpus uteri and/or adnexae# |
| IIIB∗ | Vaginal and/or parametrial involvement# |
| IIIC∗ | Metastases to pelvic and/or para-aortic lymph nodes# |
| IIIC1∗ | Positive pelvic nodes |
| IIIC2∗ | Positive para-aortic lymph nodes with or without positive pelvic lymph nodes |
| Stage IV∗ | Tumor invades bladder and/or bowel mucosa, and/or distant metastases |
| IVA∗ | Tumor invasion of bladder and/or bowel mucosa |
| IVB∗ | Distant metastases, including intra-abdominal metastases and/or inguinal lymph nodes |
| FIGO Committee on Gynecologic Oncology. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynecol Obst 2009;105:103–104. ∗Either G1, G2, or G3. ∗∗Endocervical glandular involvement only should be considered as Stage I and no longer as Stage II. #Positive cytology has to be reported separately without changing the stage. | |
Systematic pelvic and para-aortic lymphadenectomy remains one of the most important steps to assess the presence of extrauterine disease and to guide targeted postoperative treatment. GOG33 demonstrated that patients with absent or superficial myometrial invasion have a low probability of lymphatic metastases (141). Furthermore, Mariani et al. demonstrated that no patient with en-dometrioid grade 1 or 2 disease and superficial myometrial invasion harbored a lymphatic metastasis when the tumor diameter was 2 cm or less (137). The importance of tumor size as a predictor for lymphatic spread was reported by Schink et al. (142). It is possible to identify a group of pa-tients in whom lymphadenectomy is likely to increase the risk of surgical complications without producing any concrete benefits. Tumor diameter, along with myometrial invasion and histologic grade and subtype, can be utilized to determine whether or not lymphadenectomy is appropriate.
An observational study reported a significant survival benefit of para-aortic lymphadenectomy in patients at intermediate or high risk of recurrence (based on presence of histologic grade 3 or deep myometrial invasion, or lymphovascular invasion, or evidence of spread outside of the uterine corpus), compared to patients who had hysterectomy with pelvic lymphadenectomy but without para-aortic dissection. This benefit was not observed in patients with low-risk endometrial cancer (143). In addition, the Postoperative Radiation Therapy in Endometrial Carcinoma (PORTEC) studies identified patients with stage IC, grade 3 endometrial carcinoma as being at high risk of early distant spread and death when treated with hysterectomy only (no staging), followed by pelvic external-beam radiation therapy. These patients had a 31% risk of distant recurrence (136). From the literature, it seems that the patients who have the potential to benefit from surgical staging are those with risk factors such as histologic grade 3, deep myometrial invasion, or lymphovascular invasion.
In summary, surgical staging should (i) identify patients with disseminated disease who are at high risk of recurrence; (ii) target postoperative treatment; (iii) reduce the number of patients potentially requiring postoperative treatment when the provided information is used appropriately (avoiding the risk of morbidity without reasonable benefit); and (iv) possibly eradicate lymphatic disease. In spite of these potential benefits in high-risk patients, prospective randomized data demonstrating a survival advantage or reduction in overall morbidity resulting from a potential reduction of adjuvant treatment still are not available.
The FIGO published the updated surgical staging system for endometrial cancer (Table 35.6) (144). In comparison with recommendations from 1988, the new system introduces the following changes: (i) former stages IA and IB are combined; (ii) former stage IIA was eliminated so that only the presence of cervical stroma involvement is considered stage II disease; (iii) alone, peritoneal cytologic findings positive for endometrial cancer are no longer a criterion for disease upstaging (although FIGO still recommends the collection of peritoneal washing, recognizing the predictive value of positive cytologic findings when combined with other poor-prognosis factors); and (iv) stage IIIC was divided into IIIC1 and IIIC2 in accordance with the absence or presence of positive para-aortic nodes. The presence of parametrial disease is now formally recognized as stage IIIB disease.
Prognostic Variables
Although disease stage is the most significant variable affecting survival, a number of other individual prognostic factors for disease recurrence or survival are known, includingtumor grade, histopathology, depth of myometrial invasion, patient age, and surgical–pathologic evidence of extrauterine disease spread (Tables 35.7 and 35.8). Other factors, such as tumor size, peritoneal cytology, hormone receptor status, flow cytometric analysis, and oncogene perturbations, are implicated as having prognostic importance.
Table 35.7 Surgical-Pathologic Findings in Clinical Stage I Endometrial Cancer
| Surgical-Pathologic Finding | Percentage of Patients |
| Histology | |
| Adenocarcinoma | 80 |
| Adenosquamous | 16 |
| Other (papillary serous, clear cell) | 4 |
| Grade | |
| 1 | 29 |
| 2 | 46 |
| 3 | 25 |
| Myometrial invasion | |
| None | 14 |
| Inner third | 45 |
| Middle third | 19 |
| Outer third | 22 |
| Lymph–vascular space invasion | 15 |
| Isthmic tumor | 16 |
| Adnexal involvement | 5 |
| Positive peritoneal cytology | 12 |
| Pelvic lymph node metastasis | 9 |
| Aortic lymph node metastasis | 6 |
| Other extrauterine metastasis | 6 |
| Modified from Creasman WT, Morrow CP, Bundy BN, et al. Surgical pathologic spread patterns of endometrial cancer. Cancer 1987;60:2035–2041, with permission. | |
Table 35.8 Prognostic Variables in Endometrial Carcinoma
| Age |
| Histologic type |
| Histologic grade |
| Myometrial invasion |
| Lymph–vascular space invasion |
| Isthmus–cervix extension |
| Adnexal involvement |
| Lymph node metastasis |
| Intraperitoneal tumor |
| Tumor size |
| Peritoneal cytology |
| Hormone receptor status |
| DNA ploidy/proliferative index |
| Genetic/molecular tumor markers |
Age
In general, younger women with endometrial cancer have a better prognosis than older women. Two reports observed no deaths related to disease in patients with endometrial cancer diagnosed before 50 years of age (145,146). Another series demonstrated a 60.9% 5-year survival rate for patients older than 70 years of age, compared with 92.1% survival rate for patients younger than 50 years of age (147). Decreased survival was associated with an increased risk for extrauterine spread (38% vs. 21%) and deep myometrial invasion (57% vs. 24%) for these two groups. The GOG reported 5-year survival rates of 96.3% for patients 50 years of age or younger, 87.3% for patients 51 to 60 years, 78% for patients 61 to 70 years, 70.7% for patients 71 to 80 years, and 53.6% for patients older than 80 years (148).
Increased risk for recurrence in older patients was related to a higher incidence of grade 3 tumors or unfavorable histologic subtypes; however, age appears to be an independent prognostic variable. Increasing patient age appears to be independently associated with disease recurrence in endometrial cancer. In one study, the mean age at diagnosis of patients who had recurrence or died of disease was 68.6 years, compared with 60.3 years for patients without recurrence. For every 1year increase in age, the estimated rate of recurrence increased 7%. None of the patients younger than 50 years of age developed recurrent cancer, compared with 12% of patients aged 50 to 75 years and 33% of patients older than 75 years (149).
Histologic Type
Nonendometrioid histologic subtypes account for about 10% of endometrial cancers and carry an increased risk for recurrence and distant spread (150,151). In a retrospective review of 388 patients treated at the Mayo Clinic for endometrial cancer, 52 (13%) had an uncommon histologic subtype, including 20 adenosquamous, 14 serous, 11 clear cell, and 7 undifferentiated carcinomas. In contrast to the 92% survival rate among patients with endometrioid tumors, the overall survival for patients with one of these more aggressive subtypes was only 33%. At the time of surgical staging, 62% of the patients with an unfavorable histologic subtype had extrauterine spread of disease (150).
Histologic Grade
Histologic grade of the endometrial tumor is strongly associated with prognosis (132,141,149,152–156). In one study, recurrences developed in 7.7% of grade 1 tumors, 10.5% of grade 2 tumors, and 36.1% of grade 3 tumors. Patients with grade 3 tumors were in excess of fivetimes more likely to have a recurrence than were patients with grades 1 and 2 tumors. The 5-year disease-free survival rates for patients with grades 1 and 2 tumors were 92% and 86%, respectively, compared with 64% for patients with grade 3 tumors (149). Another study reported similar results, noting recurrences in 9% of patients with grades 1 and 2 tumors compared with 39% of patients with grade 3 lesions (153). Increasing tumor anaplasia is associated with deep myometrial invasion, cervical extension, lymph node metastasis, and both local recurrence and distant metastasis.
Tumor Size
Tumor size is a significant prognostic factor for lymph node metastasis and survival in patients with endometrial cancer (142,157). One report determined tumor size in 142 patients with clinical stage I endometrial cancer and found lymph node metastasis in 4% of patients with tumors 2 cm or smaller, in 15% of patients with tumors larger than 2 cm, and in 35% of patients with tumors involving the entire uterine cavity (156). Tumor size better defined an intermediate-risk group for lymph nodes metastasis (i.e., patients with grade 2 tumors with less than 50% myometrial invasion). Overall, these patients had a 10% risk for lymph node metastasis, but there was no nodal metastasis associated with tumors 2 cm or smaller, compared with 18% when tumors were larger than 2 cm. Five-year survival rates were 98% for patients with tumors 2 cm or smaller, 84% for patients with tumors larger than 2 cm, and 64% for patients with tumors involving the whole uterine cavity (137,157).
Hormone Receptor Status
Estrogen receptor and progesterone receptor levels are prognostic indicators for endometrial cancer independent of grade in several studies (158–164). Patients whose tumors are positive for one or both receptors have longer survival times than patients whose carcinomas lack the corresponding receptors. Even patients with metastasis have an improved prognosis with receptor-positive tumors (161). Progesterone receptor levels appear to be stronger predictors of survival than estrogen receptor levels, and the higher the absolute level of the receptors, the better the prognosis.
DNA Ploidy and Proliferative Index
About two-thirds of endometrial adenocarcinomas have a diploid DNA content as determined by flow cytometric analysis (162,165–174). The proportion of nondiploid tumors increases with stage, lack of tumor differentiation, and depth of myometrial invasion. In several studies, DNA content was related to clinical course of the disease, with death rates reported to be higher in women whose tumors contained aneuploid populations of cells. The proliferative index is related to prognosis.
Myometrial Invasion
Because access to the lymphatic system increases as cancer invades into the outer one-half of the myometrium, increasing depth of invasion is associated with increasing likelihood of extrauterine spread and recurrence (153,155,175). The association of depth of myometrial invasion with extrauterine disease and lymph node metastases was reported (175). Of patients without demonstrable myometrial invasion, only 1% had pelvic lymph node metastasis, compared with patients with outer one-third myometrial invasion who had 25% pelvic and 17% aortic lymph node metastases. Deep myometrial invasion (>50% for all stages; ≥66% for stage I) is the strongest predictor of hematogenous recurrence (176). Survival decreases with increasing depth of myometrial invasion. In general, patients with noninvasive or superficially invasive tumors have an 80% to 90% 5-year survival rate, whereas those with deeply invasive tumors have a 60% survival rate. The most sensitive indicator of the effect of myometrial invasion on survival is distance from the tumor–myometrial junction to the uterine serosa. Patients with tumors that are less than 5 mm from the serosal surface are at much higher risk for recurrence and death than those with tumors greater than 5 mm from the serosal surface (177,178).
Lymph–Vascular Space Invasion
Lymph–vascular space invasion (LVSI) appears to be an independent risk factor for recurrence and death from all types of endometrial cancer (178–181). The overall incidence of LVSI in early endometrial cancer is about 15%, although it increases with increasing tumor grade and depth of myometrial invasion. One study reported LVSI in 2% of grade 1 tumors and 5% of superficially invasive tumors, compared with 42% of grade 3 tumors and 70% of deeply invasive tumors (180). LVSI was demonstrated to be a strong predictor of lymphatic dissemination and lymphatic recurrence (182). Another study reported deaths in 26.7% of patients with clinical stage I disease who had LVSI, compared with 9.1% of those without LVSI (183). Likewise, an 83% 5-year survival rate was reported for patients without demonstrable LVSI, compared with a 64.5% survival rate for those in whom LVSI was present (181). Using multivariate analysis, only depth of myometrial invasion, DNA ploidy, and vascular invasion–associated changes correlated significantly with survival of patients with stage I endometrial adenocarcinomas in another report (165).
Isthmus and Cervix Extension
The location of the tumor within the uterus is important. Involvement of the uterine isthmus, cervix, or both is associated with an increased risk for extrauterine disease, lymph node metastasis, and recurrence. Cervical stromal invasion was a strong predictor of lymphatic dissemination and lymphatic recurrence, especially for pelvic lymph nodes (182). One study reported that if the fundus of the uterus alone was involved with tumor, there was a 13% recurrence rate, whereas if the lower uterine segment or cervix was involved with occult tumor, there was a 44% recurrence rate (151). A subsequent GOG study found that tumor involvement of the isthmus or cervix without evidence of extrauterine disease was associated with a 16% recurrence rate and a relative risk of 1.6 (132). Patients with cervical involvement tended to have higher-grade, larger, and more deeply invasive tumors, undoubtedly contributing to the increased risk for recurrence.
Peritoneal Cytology
Several reports noted increased recurrence rates and decreased survival rates and, on this basis, recommended treatment for positive cytology (184–186). Most of the studies included patients with other evidence of extrauterine disease spread and were performed without appropriate multivariate analysis and with patients who were incompletely staged. The GOG studycritically analyzed 1,180 clinical stagesI and II endometrial cancer patients in whom appropriate surgical and pathologic staging was performed (132). Considering only the 697 patients for whom peritoneal cytology status and adequate follow-up were available, 25 (29%) of 86 patients with positive cytology developed recurrence, compared with 64 (10.5%) of 611 patients with negative cytology. They noted that 17 of the 25 recurrences in the positive cytology group were outside the peritoneal cavity.
In contrast to these reports, an equal number of studies found no significant relationship between malignant peritoneal cytology and an increased incidence of disease recurrence in the absence of other risk factors such as extrauterine disease (186–189). Patients with positive peritoneal cytology as the only site of extrauterine disease (i.e., no adnexal or uterine serosal invasion) and without poor prognosticators (i.e., myometrial invasion more than50%, nonendometrioid histologic subtype, grade 3, lymphovascular space invasion, cervical invasion) have a very favorable outcome with an absence of extra-abdominal recurrences (190). These patients have an associated 5-year survival of 98% to 100% even when not treated with adjuvant therapy (148,191,192). On the other hand, patients with positive cytology in addition to poor prognostic factors demonstrate a high rate (47%) of distant extra-abdominal failure and may potentially benefit from systemic chemotherapy. Positive peritoneal cytology seems to have an adverse effect on survival only if the endometrial cancer has spread to the adnexa, peritoneum, or lymph nodes, not if the disease is otherwise confined to the uterus (188,189,191). These considerations led to the omission of cytology as a factor impacting stage in the FIGO 2009 staging criteria.
The following conclusions may be reached regarding the prognostic implications of positive peritoneal cytology:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


