Bruck Syndrome (OMIM 259450)
Inheritance:
Autosomal recessive.
Genetic defect:
1. Mutations in PLOD2 gene (probably located in the chromosome 17p12 region) resulting in deficiency of a bone-specific telopeptidyl lysyl hydroxylase that catalyzes the formation of cross-links in the telopeptide region of type I collagen in bone (but not ligaments or cartilage). The deficiency leads to aberrant cross-linking due to under-hydroxylation of the lysine residues [2].
2. Mutations in FKBP10 gene, encoding FKBP65, an extracellular matrix binding protein, whose mutations affect type I procollagen production [3].
Prevalence:
Very rare, only few cases reported worldwide.
Bruck syndrome (BS) is a rare, autosomal recessive disorder, with only few cases known.
Like OI (see Chap. 5), it is characterized by deformity of the spine and extremities, low bone mass, bone fragility fractures, wormian bones, and blue or white sclerae [2–4]. The main differences are the presence of congenital joint contractures—a prominent finding in BS—and the absence of the typical alterations in type I collagen that characterize OI [5].
In this disease, the rationale for using BPs is much the same as for OI: low bone mass and fragility. BPs seem to improve bone quality and strength, with fewer fractures, and to reduce bone pain.
The few published case reports have shown a positive effect of BPs. Shaheen et al. used a parenteral BP in two brothers with BS, and observed a reduction of fractures [3]. Cyclic pamidronate was successfully used by Andiran et al. to treat a boy with multiple fractures: an increase in BMD, a reduction of pain, and fewer fractures (only two during 2 years of treatment) were observed [6].
Osteoporosis-Pseudoglioma Syndrome (OMIM 259770) | |
Inheritance: | Autosomal recessive. |
Genetic defect: | Mutations (likely loss-of-function) in the LRP5 gene in osteoblasts. The LRP5 gene (probably located in the chromosome 11q13.4 region) encodes the low-density lipoprotein receptor-related protein 5 (LRP5), whose defects impair the Wnt and Norrin signal transduction [7–9]. At least 12 homozygous and 15 heterozygous mutations have been described [8–12]. |
Prevalence: | 1:2,000,000 [9]. |
The osteoporosis-pseudoglioma syndrome (OPPG) is a rare autosomal recessive disease [9]. It has some of the characteristics of moderate-to-severe OI—such as reduced bone mass, short stature, and skeletal deformity—but a major difference is the presence of congenital or infancy-onset blindness, due to the presence of a pseudoglioma. Obligate heterozygotes may have a slightly reduced BMD but do not have ocular pathology.
The affected children develop osteoporosis with vertebral and limb fractures that may cause severe pain. The rationale for BP use in OPPG is the same as for Bruck syndrome (see above) and OI. Positive effects have been observed in a few case reports. Three children (aged 9–11 years) with vertebral fractures were treated with pamidronate or clodronate for 2 years and had less pain, improved mobility, greater vertebral body size, no new fractures, and normal growth and puberty, leading to the conclusion that BPs are justified in OPPG in the presence of symptomatic vertebral fractures [12]. A 21-year-old woman treated with pamidronate had less bone pain, improved mobility, and increased BMD at lumbar spine (LS) and femoral neck (FN) [13]. Different BPs (oral risedronate, intravenous pamidronate, oral alendronate) were used in four children (aged 2–8 years) for 1.5–6.5 years: the highest increase in BMD Z-score was observed with alendronate (1 mg/kg/day) [14]. Barros et al. treated two brothers with pamidronate for 3 years and observed an increase in BMD and a decrease in fracture rate [15]. Three Turkish children, in whom three novel LRP5 mutations were discovered, were treated with BPs for 3.5–7 years and showed improved BMD Z-scores at lumbar spine, reduced bone pain, and better quality of life [16].
Homocystinuria (OMIM 236200) | |
Inheritance: | Autosomal recessive. |
Genetic defect: | Most commonly, mutations in the cystathionine beta-synthetase (CBS) gene, encoding a key enzyme of the trans-sulfuration pathway (conversion of methionine to cysteine). |
Prevalence: | 1:344,000. |
Homocystinuria is an autosomal recessive connective tissue disease, due to defects in methionine metabolism, resulting in elevated plasma levels and urinary excretion of homocysteine. The disease is characterized by mental retardation, ectopia lentis, marfanoid habitus, early-onset thrombotic vascular disease, and osteoporosis. Homocysteine reacts with many biological substances, including proteins. In particular, it may damage the glycoprotein fibrillin-1, a major component of elastin (found in arteries, cartilage, skin, the suspensory ligament of the lens, and bone). This explains the many similarities of homocystinuria with Marfan syndrome (MFS).
Homocysteine-induced bone damage (due to alterations of the growth plate during endochondral ossification) has been investigated in cellular and animal studies, but systematic studies on bone density and fractures in patients affected by homocystinuria are lacking.
Only a few case reports mention the problem of low BMD in this disease, and BP treatment has been reported in only one case, a 22-year-old woman with multiple vertebral fractures and reduced BMD (measured by dual-energy X-ray absorptiometry, DXA), who received zoledronic acid by i.v. infusion once every 12 months. No adverse effects were reported but the final results have not yet been published [17]. Like most diseases with increased bone fragility, homocystinuria may benefit from BP treatment in the presence of reduced BMD and increased fracture risk, also considering that the standard treatment with betaine has not shown positive effects on bone [18].
Fibrodysplasia Ossificans Progressiva (OMIM 135100) | |
Inheritance: | Autosomal dominant in hereditary cases; most cases due to spontaneous new mutations. |
Genetic defect: | Mutations of the ACVR1 gene. ACVR1/ALK2 (activin A type I receptor/activin-like kinase 2) is a type I receptor for bone morphogenetic proteins (BMPs). An identical heterozygous substitution of a single nucleotide (G → A) has been demonstrated in all individuals with the classic presentation of fibrodysplasia ossificans progressiva (FOP). The diagnosis can be confirmed by DNA testing (determination of the DNA sequence of the ACVR1 gene). |
Prevalence: | 1:2,000,000. Fewer than ten families with inheritance of FOP known worldwide [19]. |
FOP (formerly called myositis ossificans) is a rare but extremely disabling genetic disease of the skeletal system, characterized by the formation of extraskeletal (heterotopic) ossification within connective tissues (skeletal muscles, ligaments, tendons). The involved tissues are destroyed and replaced by bone through a process of endochondral ossification, leading to progressive restriction of mobility, particularly of the upper limbs. Heterotopic ossification can also be induced by trauma, including surgical attempts to remove the newly formed bone. The affected children appear normal at birth except for some typical malformations, such as short great toes, hallux valgus, short thumbs, and hypoplasia of digital phalanges. Skeletal malformations may also develop during embryonic development [19–24]. The diagnosis can be made even before radiographic evidence of heterotopic ossification, on the basis of rapidly appearing, painful soft tissue inflammatory swellings (flare-ups) during the first years of life, associated with deformity of the great toes [25]. The diagnosis can be confirmed by sequencing the ACVR1 gene.
FOP was the first disease in which a BP (etidronate) was therapeutically used in humans [26]. The rationale was based on the physiochemical properties of BPs as inhibitors of calcification and bone formation [1, 27]. Over some years, a few other publications reported positive results with BPs (mainly etidronate) [28, 29], but there are no recent publications.
Fibrous Dysplasia (OMIM 174800) | |
Inheritance: | None. |
Genetic defect: | Activating mutations of the GNAS gene (in chromosome 20q13), with increased expression or function of the alpha subunit of the stimulatory G protein (Gs-alpha) of adenylyl cyclase. The mutations occur in a postzygotic phase, resulting in mosaicism. The affected cells suffer from excessive production of cAMP. |
Prevalence: | ? |
Fibrous dysplasia (FD) is today the preferred name for a disease also known as osteitis fibrosa, polyostotic fibrous dysplasia, and McCune–Albright syndrome. FD is a complex syndrome, characterized by single or multiple skeletal alterations (commonly but superficially described as an overgrowth of fibrous tissue in bone, hence the name “osteitis fibrosa”), often but not invariably associated with various endocrinopathies and skin pigmentation.
In FD, bone growth and modeling are abnormal, due to localized formation of excess bone tissue, and bone remodeling is abnormally high. The result is an architecturally deficient bone, in which the organization of cortical bone, trabecular bone, and marrow space is lost, with bone deformities and fractures. In particular, in the dysplastic lesions, bone trabeculae are thinner and more numerous than normal [30].
FD develops during bone growth, and an earlier presentation usually means a more widespread disease. Thus, FD more often appears as the polyostotic form in infants, and as the monostotic form in adolescents. The ratio of polyostotic to monostotic disease is about 1:10.
The craniofacial, axial, or appendicular skeleton may be variably involved, and the disease severity depends on the number and extension of bone lesions. After puberty, new major skeletal lesions do not usually appear, but the existing lesions may still evolve. Monostotic lesions may be asymptomatic, and the diagnosis is usually made after a fragility fracture, or because of deformity or persistent bone pain. The polyostotic forms are diagnosed on the basis of a combination of pain, fracture, and deformity. In some cases there are extraskeletal complications, such as precocious puberty in females, that may appear even before any apparent skeletal involvement. Bone cysts are very common, but are different from those observed in hyperparathyroidism (brown tumors).
In FD, the high rate of bone remodeling and the production of low-quality, fragile bone are the rationale for BP use, considering its inhibitory action on osteoclasts and bone turnover.
Many patients with FD have been treated with BPs (mainly i.v. pamidronate, but also oral alendronate or risedronate), usually in combination with calcium and vitamin D supplementation, often with positive results [31]. However, Plotkins et al. reported that in seven patients treated with pamidronate for 2.2 years on average, the dysplastic bone lesions were not significantly improved [32].
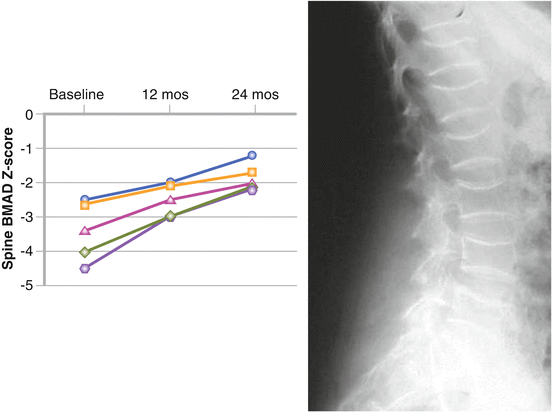
We treated two cases of polyostotic cystic fibrous dysplasia (unpublished data). The first was a 15-year-old girl sent to our attention, after her 13th fracture, by an orthopedist who had followed her for 10 years. The diagnosis had been made at 4 years of age, after a fracture of the left foot and X-ray evidence of cysts in the left leg. After a second fracture of left femur, histological examination confirmed the diagnosis. The disease was locally destructive and painful, and led to 11 further left leg fractures for minor trauma, or even spontaneous, in the following years. The clinical and radiological evolution was continuous, until cysts were present in the whole limb. BMD was always within normal range. We decided to start treatment with pamidronate (0.8 mg/kg i.v., once a month, then 30 mg every 2 months). The girl was successfully treated for 4 years, with resolution of bone pain, no new fractures, and stabilization of the cystic lesions at MRI (Figs. 6.1 and 6.2). Our second case was a 23-year-old young lady affected by polyostotic cystic fibrous dysplasia localized in the frontal-parietal area of the skull. She suffered from frequent headaches and presented with moderate left exophtalm and a wide area of softened or destroyed cystic cranial bone, where pulsating endocranial structures could be felt. We started i.v. neridronate, 50 mg every month at first, then every 2 months. After 4 years of treatment, she had no more headaches, the exophtalm was significantly improved, and MRI demonstrated reduction of the cystic bone area.
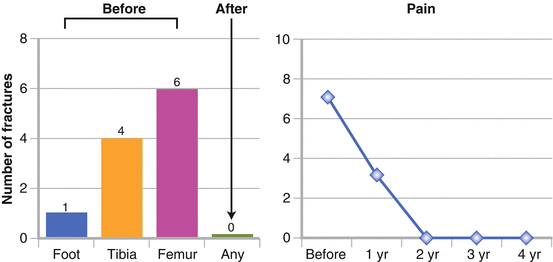
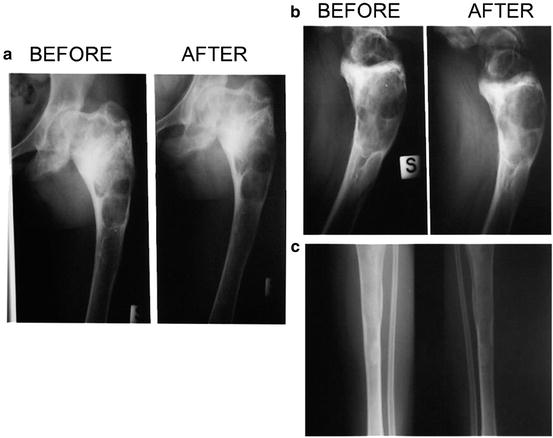

Fig. 6.1
A 15-year-old girl affected by polyostotic cystic fibrous dysplasia interesting the whole left lower limb, with a history of multiple fractures. Effect of pamidronate therapy on number of fractures and bone pain

Fig. 6.2
(a–c) MRI images of the left lower limb in the same patient before and after pamidronate, showing stabilization of the cystic lesions
Trisomy 9p (OMIN 190685) | |
Inheritance: | In some cases, due to balanced chromosomal rearrangement in one of the parents; in others, arising from de novo errors in early embryonic development. |
Genetic defect: | Duplication of the short arm of chromosome 9. |
Prevalence: | 200 cases reported worldwide. |
Trisomy 9p is a rare chromosomal abnormality, first described by Rethoré in 1970 [33]. Unbalanced translocation t(9;14) (i.e., trisomy of the short arm of chromosome 9 and monosomy of the short arm of chromosome 14) is a rarer variant. These anomalies are compatible with long survival. The clinical manifestations are very variable: mental retardation, short height, prominent or bulbous nose, down-turned corners of the mouth, hypertelorism, strabismus, and foot and hand anomalies are most often described [33–36]. Only delayed bone maturation has been reported [35], but not low BMD and fragility fractures.
We personally observed both low BMD and fragility fractures (osteoporosis according to the ISCD PPDC 2013 definition) in two Italian children with unbalanced translocation t(9;14), referred to our institute (Fig. 6.3). The first was a 12-year-old boy who had sustained two subsequent fractures after minimal trauma, and the second was a 6-year-old girl with multiple, apparently atraumatic, vertebral fractures. In both cases, DXA revealed very low lumbar spine BMAD Z-scores (−2.5 and −3.0, respectively),2 and we decided to start treatment with i.v. pamidronate (0.5 mg/kg, for 3 consecutive days every 3 months). After, respectively, 8 months (boy) and 2 years (girl) of treatment, both had a significant BMAD increase (+8.9 %, boy; +11.4 %, girl) and, above all, no more fractures. As often happens in the growing age, the girl also showed a good recovery in height of the crushed vertebral bodies (unpublished data).
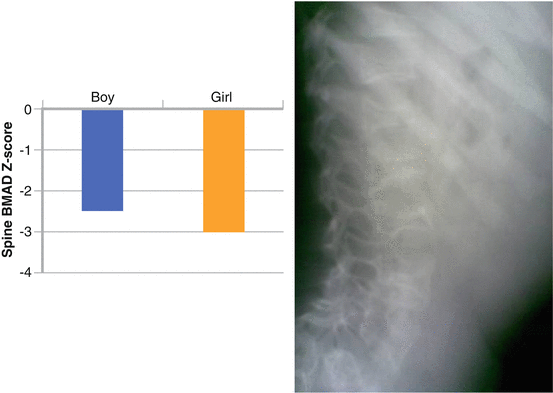

Fig. 6.3
Lumbar spine BMAD Z-scores in boy and girl affected by unbalanced translocation t(9;14) (left). Multiple vertebral fractures in the girl before pamidronate therapy (right)
More Frequent Diseases
Ehlers–Danlos Syndrome (OMIN 13000–13050) | |
Inheritance: | Autosomal dominant; autosomal recessive; due to spontaneous new mutations. |
Genetic defect: | Various defects in extracellular matrix proteins. Gene mutations described for ADAMTS-2, COL1A1, COL1A2, COL3A1, COL5A1, COL5A2, PLOD1, and TNX-B. |
Prevalence: | 1:5,000. |
Ehlers–Danlos syndrome (EDS) refers to at least nine different genetic disorders of connective tissues, caused by defects in one of the genes encoding extracellular matrix proteins (including collagens and small leucine-rich proteoglycans like decorin). The main clinical features are skin hyperextensibility, joint hypermobility, and tissue fragility of the skin, ligaments, blood vessels, and internal organs.
Yen et al. measured BMD in 11 patients with EDS (children, adolescents, and young adults) and observed osteoporosis in all of them [37]. Dolan et al. reported an increased fracture rate in EDS patients [38]. These findings suggest reduced bone strength, but further studies on larger patient samples affected by the various EDS forms are needed to assess the actual impact of this disease on bone. There are no published data on BP use in EDS. However, if the presence of reduced BMD and increased fracture rate are confirmed, treatment with BPs might improve the condition and should be investigated.
Marfan Syndrome (OMIN 154700) | |
Inheritance: | Autosomal dominant. |
Genetic defect: | Mutations in FBN1 gene, one of the two genes encoding fibrillin-1 (the main structural component of elastin-associated cross-links). Rare mutations in COL1A2 gene. Rare mutations in TGFBR2 gene, encoding transforming growth factor beta (TGF-beta) receptor 2. |
Prevalence: | 1:5,000. |
MFS is a relatively common autosomal dominant genetic disease, variably affecting the skeleton, eye, and cardiovascular system. The most characteristic clinical feature is long, thin, hyperextensible fingers (arachnodactyly). The most common gene mutations (FBN1) lead to an increased activation of TGF-beta, which stimulates osteoblast proliferation and differentiation, influencing bone mass and the properties of bone matrix [39]. Some studies evaluated BMD in adults and children affected by MSF: the findings are inconsistent for children, reporting reduced as well as normal BMD, while both reduced BMD and increased fracture risk are observed in adults [40–42]. These features may constitute the rationale for BP treatment, at least in selected cases. Currently, there is only one published study that used alendronate in an animal model, Fbn1 (mgR/mgR) mice with severe MFS and osteopenia [43]. These mice have normal osteoblast differentiation and bone formation, but excessive osteoblast-stimulated pre-osteoclast differentiation and increased osteoclastogenesis, leading to osteopenia. Alendronate treatment improved bone quality by reducing osteoclast activity, but had no effect on aneurysm progression.
Duchenne Muscular Dystrophy (OMIN 310200) | |
Inheritance: | X-linked recessive. |
Genetic defect: | Mutation in the dystrophin gene (at locus Xp21, in the short arm of chromosome X). |
Prevalence: | 1:3,500 male births. |
Duchenne muscular dystrophy (DMD) is the most frequent muscular disease affecting children. In most cases, proximal muscle weakness begins before 3 years of age, then patients become progressively unable to walk. Notwithstanding substantial therapeutic advances, essentially due to early treatment with glucocorticosteroids (GCs), most patients still die in early adulthood.
The presence of low bone mass, mainly due to both physical inactivity and GC treatment, has been frequently reported in DMD. Fractures are a common complication and a major factor in the precocious loss of independent ambulation [44]. The few published studies on bone density in DMD concluded that BMC and BMD were lower than normal at different skeletal sites [45–51], particularly at lower limbs [47, 49]. In a retrospective study on 143 DMD boys, the GC-treated subjects had 2.6 times more long-bone fractures than the untreated; and vertebral fractures occurred in 32 % of GC-treated patients, versus none in the untreated [45]. In a study on 33 boys with DMD, treated with GCs for 100 months, 24 sustained vertebral fractures [46]. In 46 boys with DMD, treated with deflazacort for 4 years, 26 suffered 37 fractures (14 vertebral) and significant BMD decrease was observed [47]. A recent study on 25 boys (mean age 7.4 years), however, did not show any detrimental effects of GCs (given for 30 months) on lumbar vertebrae [48].
The use of BPs in children affected by DMD is justified by the increased fracture rate due to low BMD (cytokine-induced increased osteoclastogenesis and disuse osteopenia) and long-term GC treatment, a well-known cause of osteoporosis. Different BPs (pamidronate, alendronate, risedronate) have been used in DMD boys on long-term GC treatment. The few published studies consistently report increases in BMD and fewer fractures. In 23 deflazacort-treated boys, with only a slight decrease of the BMD Z-score and no fractures, alendronate treatment plus calcium and vitamin D supplements for 2 years improved total body and lumbar spine BMD Z-scores [52]. In three patients with reduced BMD Z-score, fractures, and generalized bone pain, oral alendronate (10 mg/day, for 14–25 months) was well tolerated, without adverse effects, and led to increased lumbar spine BMD, reduction of bone pain, and no incident fractures [53]. A recent retrospective study on a cohort of 44 Canadian patients, treated with GCs (prednisone or deflazacort) at a single center, even reported that BP treatment was associated with a significant improvement in survival rate, compared with treatment with steroids alone. This interesting finding should however be confirmed by larger studies [54]. We have been using BPs (i.v. pamidronate or oral alendronate) in children and adolescents with DMD and osteoporosis (i.e., BMD Z-score less than or equal to −2 and a history of fragility fractures) in the last 4 years, and observed improvements in BMD and fewer fractures (Fig. 6.4) (unpublished data).