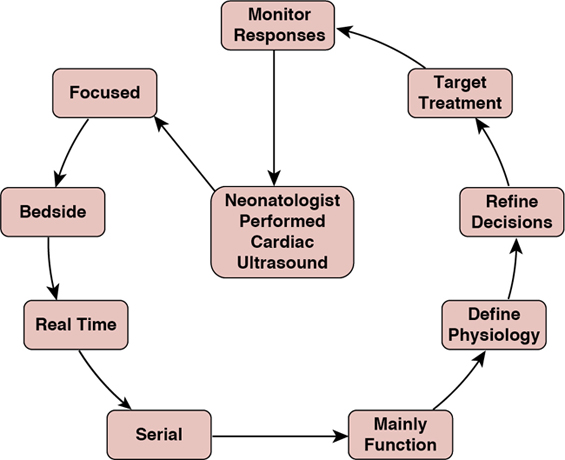
Nicholas Evans, Martin Kluckow, Audrey Hébert Key points It is a limitation in the intensive care of the newborn infant that we have few tools with which to monitor cardiovascular and hemodynamic function. The mainstay has been to continuously monitor invasive blood pressure and heart rate. Beyond that, reliance is placed on rather inaccurate measures of contemporaneous hemodynamic well-being such as skin capillary refill time and urine output and measures that do not reflect the changes in the hemodynamic status in real time such as the acid-base status. Near-infrared spectroscopy (NIRS) is evolving as a potentially useful monitoring tool1 for the real-time assessment of tissue oxygenation of the organ interrogated (brain, kidney, muscle, or intestines). As for measuring cardiac output, there is little outcome-based validation for tools designed to measure cardiac output (Chapters 14 and 21). The difficulties of small patient size and a smaller commercial market, limit neonatology’s access to a range of tools for monitoring cardiac output that are available for the intensive care of the older subject. These tools include thermodilution, electrical impedance velocimetry, continuous Doppler methodologies, and derivations from blood pressure waveforms. Doppler cardiac ultrasound provides a noninvasive, albeit non-continuous, technique from which it is possible to derive estimates of a wide range of hemodynamic parameters as well as information on structure and function of the organ that drives the circulation, the heart. Integration of cardiac ultrasound into routine neonatal intensive care has been limited by the concentration of the necessary skills in specialist groups who work predominantly outside the NICU. This results in information that is often neither timely nor particularly well focused on hemodynamics. From a research perspective, it meant that there were assumptions derived from a limited number of snapshots rather than serial studies to document natural history. Neonatology, like many acute care specialties, has recognized the limitations of an external consultative ultrasound model and neonatologists are increasingly developing cardiac ultrasound skills themselves so that it can be applied at the acute point of care.2 This has allowed more systematic serial studies, which in turn is facilitating the development of the research agenda and the clinical monitoring potential of these methodologies. It should be emphasized that acute CPU does not replace the need for consultative ultrasound. Rather it addresses different questions and complements consultative ultrasound. The best outcomes are achieved when the two models work collaboratively alongside each other. This chapter will provide an overview of the use of cardiac ultrasound to assess the neonatal circulation (details of the individual techniques will be outlined in other chapters) as well as discuss issues of training, accreditation, and interaction with other imaging specialists. The evolution of ultrasound into the area of acute care has resulted in political controversies, and this has not been confined to neonatology. On one side of the argument, acute care specialists have recognized the rapid diagnostic potential of ultrasound, a potential that is difficult to fulfill within a consultative model of ultrasound.3 The improvement in quality of imaging and portability of equipment has made ultrasound accessible to anyone with a good understanding of anatomy and access to training. On the other side of the argument, some consultative imaging specialists have resisted this on the basis of concerns about lack of accredited training and about the risk of diagnostic error.4 Importantly, a review of medico-legal cases involving neonatal/pediatric CPU in the United States found no evidence of this risk5 and several more recent case series show excellent anatomical concordance between cardiologists and appropriately trained CPU practitioners. Like all political divides, there are merits to both arguments. Consultative ultrasound specialists have neither the ability (nor often the desire) to be available 24/7 for the rapid diagnostic situations facing acute care specialists. CPU, particularly in neonatology, has often evolved with a lack of good training and accreditation structures. Many of the early adopters of CPU were largely self-taught or taught by a cardiologist with a balanced perspective of the usefulness of functional ultrasound in the NICU. While this is changing, as will be described later, there is risk that this may lead to diagnostic error. As always, the answer lies in the middle, with a system that takes advantage of the strengths of both systems and minimizes the risks. Clinician-performed ultrasound (CPU) is usually a predominantly “rule-in” diagnostic modality and does not aim to “rule out” all diagnoses, whereas consultative ultrasound should also be viewed as a “rule-out” diagnostic modality. From our experience, much of the resistance to establishing a CPU program is based on a lack of understanding of the goals. Resolution comes from reassurance about training and quality issues and education about purpose, most importantly, that the intent is to complement, not replace, the role of those consultative specialties. It is the nature of the evolution of any procedure or technology that the early adopters are often self-taught. Development of training and accreditation structures follows when the need is identified and there is enough of a critical mass of early adopters to provide the necessary support. There are good examples of training structures in CPU such as that developed by the American College of Emergency Physicians6,7; however, there is a long way to go in neonatal CPU. Internationally, there is difference of opinion in the relative weight that should be given to training provided by those outside neonatology compared to those within. In Australia and New Zealand, we have developed a training program that is based on the philosophy that training for CPU in the NICU is best undertaken in the NICU under supervision of appropriately trained and accredited neonatologists, while also recognizing the need for support from the consultative imaging specialists.3 The program was developed in 2007 under the auspices of the Australasian Society of Ultrasound in Medicine (ASUM), who, having recognized the inevitable evolution of ultrasound into acute care areas, had developed a qualification called the Certificate in Clinician Performed Ultrasound (CCPU). There were already modules for several acute care specialties and neonatology was developed as another module. The program was developed by a steering committee consisting of mainly neonatologists from around Australia and New Zealand but included a radiologist and a pediatric cardiologist. The qualification is based on course attendance and supervised logbooks of ultrasound scans. The trainees have to complete an online physics course, following which the ultrasound training is in two stages, basic and advanced. Basic training is aimed at normal image acquisition of the heart and brain, while advanced training is aimed at interpretation of abnormal hemodynamic and cerebral findings, as well as learning some other aspects of neonatal CPU such as basic abdominal organ imaging and central line localization. The core part of training is undertaken within the neonatal unit under the supervision of a qualified CPU clinician who can also teach the skills of integration of the ultrasound and clinical findings. The advanced module includes training in recognition of common congenital heart disease with the understanding that the ability to exclude congenital heart disease will require further training under the supervision of pediatric cardiology. The expectation is that only a few neonatologists undertake this extra training and others will continue to consult with pediatric cardiology colleagues to exclude structural cardiac abnormalities. This program has been running since 2007; there are more than 60 graduates with the CCPU (neonatal) and approximately 40 trainees currently undertaking training.8 Consensus statements on neonatal cardiac ultrasound training have been published in North America, Europe, and the UK.9,10 Both these statements take a different focus, with an emphasis on training within pediatric cardiology. Neither of these statements addresses the wider role for neonatal CPU beyond the heart. The development of national or regional administered structured training programs under these guidelines is still in progress. It is our view that to achieve the goal of quality assurance in standards of neonatal CPU, a training program needs to be both relevant and workable. For this to happen, the practical component of the training needs to occur mainly within the NICU and the training needs to be mainly provided by neonatologists. A core component of training is understanding how to integrate imaging skills into the other clinical information available for a particular patient. Further, the training program needs to embrace the fact that CPU in any acute care specialty embraces more than one organ. The concept of neonatal hemodynamics has received broad adoption with the establishment of new programs across North America and in many parts of the world.11 The impetus for the growth of these programs is the need for rapid evaluation, an individualized approach to neonates with hemodynamic disturbances, and the call for standardization through research. Program structure and quality assurance are an integral part of the hemodynamic consultation process. In addition, access to dedicated echocardiography equipment, systems for study archiving and report generation, safety, quality assurance, and infection control practices are essential components of successful programs. In centers with Pediatric Cardiology services, close collaboration on operational, training, and research matters is encouraged. The field has grown exponentially in the past decade, particularly in North America, with the establishment of neonatal hemodynamics programs in more than 20 centers. Importantly, the concept of Targeted Neonatal Echocardiography (TNE) and neonatal hemodynamics has been positively embraced by major societies such as the American Echocardiography Society (ASE); specifically the establishment of the Neonatal Hemodynamics and TNE Specialty Interest Group (NHTS SIG) provides an avenue for cultivation of knowledge and scientific discovery and a path for collaboration between disciplines. Finally, the creation of academic consortia, such as the Pan American Hemodynamics Collaborative, and the establishment of a Neonatal Hemodynamics Research Center (https://neonatalhemodynamics.com) enable clinical, educational, and academic oversight of the field and the development of formal mentorship processes. The implementation of neonatal hemodynamics programs has also influenced the development of training programs. The training model for neonatal hemodynamics requires exposure to a broad range of illnesses in both term (e.g., pulmonary hypertension, hypoxic-ischemic encephalopathy, surgical disease) and preterm infants. The curriculum should include extensive “hands-on” training (image acquisition and measurement analyses) and “cognitive” (advanced cardiovascular physiology, core pharmacology, and pathophysiology) components as they apply to common neonatal cardiovascular health problems. Although training in the echo lab, as suggested in the ASE 2011 guidelines,12 can provide additional exposure to congenital cardiac malformations that trainees should recognize, the lack of access to pediatric echocardiography laboratories in many regions may represent an obstacle to establishing training programs. It is important to highlight, however, that the primary goal of the neonatologist with hemodynamics expertise is to recognize deviations from normal anatomy and appropriately refer to a cardiologist. Therefore most training may take place within the NICU and be supervised by neonatologists with expertise in neonatal hemodynamics. Trainees gain image acquisition skills within the NICU through graded exposure to a variety of neonatal cardiovascular health problems, and time in the echo lab is often integrated into the training program. Review sessions, collaborative discussions with pediatric cardiology, participation in special interest groups in neonatal hemodynamics, and research are all fundamental components of training. A singular approach to training may not be achievable and regional or international variance must be allowed for developing training guidelines. The development of echocardiography simulators and web-based APPs provides a novel opportunity to modernize learning. Standardized imaging protocols for the diagnosis and longitudinal evaluation of common neonatal problems are desirable. These protocols may include evaluation of the hemodynamic significance of patent ductus arteriosus (PDA), evaluation of pulmonary hemodynamics and right heart function (e.g., acute or chronic pulmonary hypertension), and evaluation of left heart function and systemic hypoperfusion (e.g., post-PDA ligation, septic shock). The incorporation of advanced echocardiography techniques (e.g., tissue Doppler imaging, strain analysis) into standardized imaging protocols based on new knowledge requires thoughtful consideration. The growth of the field of Neonatal Hemodynamics represents a natural evolution to further optimize clinical care and scientific knowledge in a subpopulation of critically ill preterm and term infants with intrinsic developmental vulnerability, which places them at increased risk of disease-dependent hemodynamic instability and cardiopulmonary maldevelopment. The publication of the American Society of Echocardiography (ASE) Guidelines for TNE in 201112 was a pivotal step in cultivating the growth of this expert model of hemodynamic care. The subsequent 10 years have witnessed major expansion in North America in terms of new clinical programs, scientific advancement, and innovation. Importantly, a key component of TNE and one of the primary differences compared with traditional echocardiography is that performance of the imaging and study interpretation by the neonatologist enables enhanced integration within the intensive care context. Appraisal of physiology-based echocardiography requires detailed knowledge of the modifiers of neonatal physiology, such as ventilation, fluid balance, and other important variables. The concept of a “hemodynamic consultation” was proposed to encompass the comprehensive, integrated assessment by a neonatologist with advanced echocardiography skills and a strong foundation in neonatal pathophysiology and neonatal cardiovascular pharmacotherapeutics.13 Indications for hemodynamic consultation may include “disease-specific” evaluation such as hemodynamic significance of patent ductus arteriosus (PDA), assessment of pulmonary hemodynamics and right heart function (e.g., acute or chronic pulmonary hypertension), assessment of left heart function and systemic hypoperfusion (e.g., post-PDA ligation, septic shock), or “symptom-based” evaluation such as systemic hypotension or hypoxemia. As such, the assessment is dependent on the knowledge of cardiovascular physiology and pharmacotherapeutics, and the ability to integrate this information. The hemodynamics consultation process may be summarized into three major phases: A key use of CPU in the clinical setting is to help understand the underlying physiology and subsequently to decide on what treatment is most useful for the individual patient. An illustration of the process of identifying individual physiology in a focused ultrasound, applying this information, and assessing the outcome of treatment choices is shown in Figure 9.1. This targeting of treatment with the ability to longitudinally monitor response is the way we most often use CPU and is best demonstrated by some case presentations.
Chapter 9: Ultrasound-guided hemodynamic management
Introduction
Politics of ultrasound
Training and accreditation in Australia/New Zealand
Training and accreditation in North America
Moving toward the hemodynamic consultation
Integrating clinical and physiological information to target treatment
Ultrasound-guided hemodynamic management