(1)
Department of Fetal Medicine and Obstetric & Gynecological Ultrasound, Manipal Hospital, Bangalore, Karnataka, India
Ovaries are bilateral ovoid masses on either side of the uterus that have a dual function of producing hormones and ova. Each ovary is anchored in the pelvis by the mesovarium to the posterior surface of the broad ligament, by the utero-ovarian ligament to the uterus and by the suspensory ligament to the lateral pelvic wall. These ligamentous structures are lax and not rigid, and consequently the position of the ovaries varies in different patients and in the same patient at different times.
7.1 Evaluation of Ovaries and Persistent Adnexal Masses
7.1.1 Morphology, Measurement and Doppler Evaluation of the Ovary and Ovarian Masses (Including Persistent Adnexal Masses)
The ovaries (Fig. 7.1) are identified in the reproductive age group by the presence of follicles. They are typically located just medial to the external iliac vessels and anterior to the internal iliac vessels. Therefore, on a transverse section, the external iliac vessels lie lateral to it, and on a longitudinal section, the internal iliac vessels lie posterior to it. Most often, the ovaries are easy to locate on either side of the uterus, but at times this can be challenging. This is more likely in postmenopausal women with atrophic (small in size and without follicles) ovaries. The ovaries can usually be located on ultrasound by angulating the probe from a TS view of the uterus along the hypoechoic utero-ovarian ligament from one cornu to the lateral pelvic wall. Another method to locate the ovary is to trace along the external iliac vessels in a transverse section of the pelvis. The normal ovary in a woman of reproductive age group shows a few antral follicles. Antral follicles are seen as small anechoic cysts in the ovary, 2–9 mm in diameter. A developing follicle or corpus luteum (CL) may also be seen, depending on the phase of the menstrual cycle. Details of assessing developing follicles and corpus luteum are given in the section on follicular tracking in Chap. 13. The ovaries are measured in all cases. Their mobility is also assessed by applying pressure with the TVS probe. Normally the ovaries are mobile structures and slide along or can be made to move away from the uterus and lateral pelvic wall. However, they could be adherent to the uterus or the lateral pelvic wall in the presence of pathological conditions like endometriosis.
If there is an ovarian mass, then the ovary is evaluated further in detail as given below, primarily based on IOTA recommendations.

Fig. 7.1
Normal ovaries. (a) Ovary with a developing follicle. External iliac vessels (arrow) are seen just lateral to the ovary. (b) The ovary shows a corpus luteum. External iliac vessels (arrow) are seen lateral to the ovary. (c) The ovary shows antral follicles (arrows)
Crescent sign (Fig. 7.2): An ovarian mass may show ovarian tissue adjoining and stretched around it, called the ‘crescent sign’, which helps in determining its ovarian origin. In large masses, the ovarian tissue may be completely compressed, such that no ovarian tissue is identifiable around it.

Fig. 7.2
Crescent sign – crescent-shaped ovarian tissue seen adjacent to the ovarian cyst (arrow), which is stretched around the cyst as if it were hugging the cyst
Solid component (Fig. 7.3): The presence of a solid tissue increases the likelihood of malignancy. Doppler flow helps to confirm the presence of solid tissue. The solid tissue may be present along the margins of the mass or may be present within the mass. Some ovarian masses may be completely solid. The presence of a papillary projection (as described later) is also considered a solid component of a cyst. The absence of solid tissue in a mass (i.e. a completely cystic mass) increases the likelihood of it being benign.

Fig. 7.3
(a) No solid component. (b) Completely solid mass. (c) Solid area (arrow) along the outer margins of the mass. (d) Solid area (arrow) within a predominantly cystic mass
Papilla (Fig. 7.4): Any solid projection into the cavity from the cyst wall, with a height of at least 3 mm (from the inner margin of the cyst), is considered a papilla. The presence of a papilla increases the likelihood of malignancy.
Any solid projection of less than 3 mm is considered an irregularity in the cyst wall. If the maximum diameter of the largest solid component or papilla is less than 7 mm, then there is a high probability that the mass is a benign cystadenofibroma.

Fig. 7.4
(a) No papilla seen. (b) Single papilla seen with height of papilla measured in the image. (c) Multiple irregular papillae seen along the septae. (d) Maximum diameter of papilla is less than 7 mm in this case of a cystadenofibroma
Septum and a number of locules (Figs. 7.5 and 7.6): A septum is a strand of tissue originating from one wall of the cyst to the other. The correct way to measure the septum is when it is perpendicular to the ultrasound beam. Septae of less than 3 mm are more commonly seen in benign cysts, whereas septae which are more than 3 mm and irregular are more commonly associated with malignant masses.

Fig. 7.5
(a) Thin septum (arrow). (b) Thick slightly irregular septum (arrow)
In the absence of septum, a cyst is considered unilocular. A cyst with one or more septae is considered multilocular. The locules may vary from 2 to as many as 100. An incomplete septum as seen in a hydrosalpinx is not considered a true septum, and the cystic mass is still considered unilocular.
Unilocular cysts are more likely to be benign.

Fig. 7.6
(a) Unilocular cyst. (b) Multilocular cyst
Walls of the mass (Fig. 7.7): The more irregular the inner walls of a cystic mass or the external walls of a solid mass, the more likely it is to be malignant.

Fig. 7.7
(a) Solid tumour with regular, smooth, outer margins. (b) Solid tumour with irregular, lobulated, outer margins. (c) Smooth inner walls of a cystic mass. (d) Irregular inner walls of cystic mass with multiple papillae
Contents of a cyst (Fig. 7.8): The contents of a cyst are not useful in assessing the likelihood of malignancy. However, it is often useful in the characterisation of the mass into various histopathologies. The cyst may be anechoic, show low-grade internal echoes, be hypoechoic with a ground glass appearance or show mixed echoes with hypoechoic and hyperechoic contents. The cystic contents may show fluid–fluid level.

Fig. 7.8
Contents of a cyst: (a) anechoic, (b) with low-grade internal echoes, (c) hypoechoic, (d) with fluid–fluid level, (e) with mixed echoes
Acoustic shadowing (Fig. 7.9): Loss of echoes beyond a sound-absorbing structure is called acoustic shadowing. It is seen typically in dermoids, fibromas and behind the papillae of a cystadenofibroma. The presence of acoustic shadowing increases the likelihood of the mass being benign significantly.

Fig. 7.9
Acoustic shadowing in (a) dermoid, (b) fibroma, (c) papilla of a serous cystadenoma fibroma. Shadows are outlined in the images
Lesions and ovaries – The size of both the ovaries and any lesion should be measured in three perpendicular dimensions (x, y and z) in two perpendicular planes and given in millimetres (as explained in Chap. 2). From this, the volume of the mass can be calculated by the formula ‘x × y × z × 0.523’.
At times, the cyst is very large and its entire margins cannot be visualised in a single screen. One can increase the sector angle and depth. However, with very large masses, even this may not suffice. In such cases, in order to approximately measure these masses (particularly the length of the mass per abdomen which may be difficult), one can resort to a dual image on screen, so that one margin of the mass to beyond the centre of the mass is captured in the image on one half of the screen and the opposite margin to beyond the centre of the mass is captured in the image on the other half of the screen. The length of the mass is measured in two parts. One measurement is taken from one margin to some common reference point closer to the centre in one image and the second, from the same reference point in the second image to the other opposite margin of the mass. The final length is the sum of these two measurements. A large cyst can also be measured using the panoramic imaging mode, available in some ultrasound machines.
Septum (Fig. 7.5) – The septum is measured where it appears to be the thickest (not close to its attachment to the internal cyst wall). It is preferable to measure a septum with the ultrasound beam perpendicular to it.
Papilla – The largest projection is measured in three dimensions, in two perpendicular planes (height, base and base). The number of separate papillary projections should also be noted. When in doubt about the number of papillae, particularly when they appear to merge with one another, it is recommended that the worst-case scenario should be applied. For example, if there is a doubt of 3–5 papillae, it is better to consider it as 5 papillae.
The number of locules in the tumour is also assessed (1 to 10 and more than 10).
The largest solid component should be measured in three orthogonal diameters in two perpendicular planes. This applies to papillary projections also, if it is the largest solid component.
Fluid in POD – It is measured in a sagittal section of the uterus, and the largest AP measurement is noted in millimetres.

Fig. 7.10
Cyst measured in the three largest dimensions in two perpendicular planes. When ovarian tissue is not seen around the cyst, the measurement of both the cyst and the ovary is the same

Fig. 7.11
Measuring the length of a large cyst. (a) Here the mass is imaged on a split screen as it is too large to fit in a single screen. The length of the mass is measured using a common reference point (arrows). (b) A large cyst can also be measured using the panoramic imaging mode, available in some ultrasound machines
Doppler study of the ovarian mass: The entire tumour should be examined by Doppler imaging. Flow may be seen in the walls, septae, solid areas and papillae. Flow within solid areas and papillae is, however, more often associated with malignancy. For Doppler flow assessment, settings must be optimised. Generally, the PRF should be set at 0.3 for colour Doppler and 0.6 for power Doppler (in some machines, this is equivalent to velocity scale of 3–6 cm/sec). Thereafter, the Doppler gain should be increased until artefacts appear, following which gain should be gradually reduced to when the artefacts just disappear. Ultrasound frequency should be at least 5.0 MHz and wall filter should be between 30–50 Hz. It is useful to have preadjusted Doppler settings for gynecological scans to save time and for appropriate evaluation.
Colour index (Fig. 7.12): The most accepted method of assessing Doppler flows is colour scoring, a subjective semi-quantitative evaluation where scores are given from 1 to 4:
1 – no flow
2 – minimal flow (few colour spots)
3 – moderate flow
4 – abundant flow
The absence of flow (colour score 1) increases the likelihood of the mass being benign. Colour score of 3–4 increases the likelihood for malignancy but may also be seen in benign adnexal masses, infections, corpus luteum and trophoblastic tissue.
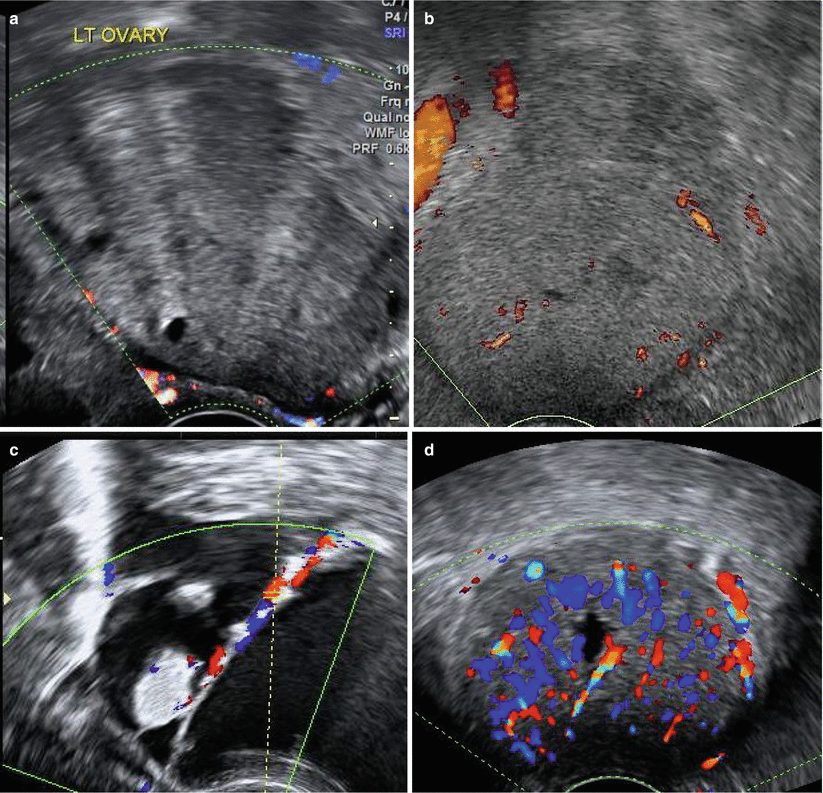
Fig. 7.12
Colour score: (a) Score 1 – no flow. (b) Score 2 – minimal flow. (c) Score 3 – moderate flow. (d) Score 4 – abundant flow
Flow indices (Fig. 7.13): When several samples are taken, the set of results with the highest peak systolic velocity (PSV) and the corresponding values of PI and RI are selected. Malignant masses generally show flow with low resistance, i.e. an RI of less than 0.4 and PI of less than 1.0. However, a significant overlap is seen between benign and malignant masses with low RI seen in only 25 % of malignant lesions. This is, therefore, less helpful in diagnosis.

Fig. 7.13
Low-resistance flow in (a) malignant mass (fallopian tube carcinoma), (b) benign mass (fibroid)
Colour distribution: The location of flow may also help in assessing the possibility of malignancy. Malignant masses are more likely to have central flow, while in benign ones, flow is more likely to be peripheral. However, there is a significant overlap noted.
7.1.2 Morphological Classification of Ovarian/Adnexal Masses (Fig. 7.14)
As per IOTA recommendations, all adnexal masses are classified into one of the five types mentioned below, based on greyscale imaging. The likelihood of malignancy for each of these types is also mentioned in the table below.
Timmerman et al. UOG (2008)
Type | Malignant (%) |
|---|---|
Unilocular (no solid areas or papillae) | 1.3 |
Multilocular (no solid areas or papillae) | 10 |
Unilocular solid a | 37 |
Multilocular solid a | 43 |
Solid b (80 % or more of the tumour appears solid) | 65 |
The presence of a solid tissue, as seen in this table, increases the likelihood of malignancy significantly. The more heterogeneous and irregular the solid area, the higher is the likelihood of malignancy. Also, central flow in a solid area or a papilla increases the likelihood of malignancy.


Fig. 7.14
Morphological classification of ovarian neoplasms: (a) unilocular, (b) multilocular, (c) unilocular solid, (d) multilocular solid, (e) solid
7.2 Normal Ovaries
Ovaries vary in size and appearance not only based on the age of the individual but also through the menstrual cycle due to hormonal influences.
Neonatal Ovaries
(Fig. 7.15) The ovaries are located above the true pelvis and, therefore, easily seen on TAS. They move deeper into the pelvis as the child grows. At birth, the typical volume of the ovary is about 1 cc but may reach up to 3.5 cc due to the abrupt rise in FSH following a decrease in oestrogen and progesterone that reaches the fetus in intrauterine life from maternal circulation. Ovarian follicles have been noted as early as 28 weeks of gestation, and follicles of less than 9 mm may be seen in neonatal ovaries. Occasionally, the development of larger physiological cyst (more than 1 cm) may be seen in both fetal and neonatal ovaries and most resolve spontaneously. About 20 % of neonates have ovarian cysts of more than 1 cm.


Fig. 7.15
Neonatal ovaries in a 32-day-old baby who was on follow-up for a right ovarian cyst detected antenatally. (a) Haemorrhagic right ovarian cyst of 27 cc showing debris in its posterior dependent part (arrow). (b) Multicystic left ovary measuring 2.8 × 1.4 × 1.8 cm (volume 3.8 cc). This (multicystic ovary) is seen in some infants, secondary to rise in FSH due to a sudden fall in oestrogen and progesterone levels that were present at higher levels in neonatal life from maternal circulation. Uterus measured 3.0 × 1.1 × 1.3 cm
Paediatric Ovaries
(Fig. 7.16) The ovarian volume up to the age of about 7 years is about 0.5–1.5 cc increasing to 4 cc just before puberty. Larger physiological cyst (more than 1 cm) may be seen in prepubertal ovary. Ovarian volume of more than 4 cc and the presence of six or more follicles in girls younger than 7 years should raise the suspicion of precocious puberty.


Fig. 7.16
(a–c) Case 1 – Paediatric ovaries in a 6-year-old girl. (a) The uterus measures 3.7 × 1.0 × 1.6 cm. (b) Right ovary 1.2 × 0.6 × 1.5 cm. (c) Left ovary 1.3 × 0.7 × 1.5 cm. No follicles are seen in either ovary. (d–f) Case 2 – Prepubertal ovaries in an 11-year-old. (d) The uterus measures 3.5 × 1 × 1.2 cm. (e) Right ovary 2.3 × 1.1 × 2.6 cm. (f) Left ovary 1.3 × 0.7 × 1.5 cm. Follicles are seen in both ovaries
Reproductive Ovaries (Fig. 7.17)
The ovarian volume may vary from 4 to 20 cc with an average of about 5–6.5 cc. Developing and immature (antral follicles 2–9 mm) can be seen throughout the entire cycle and appear as unilocular, anechoic well-defined cysts. In the first half of the menstrual cycle, one or more dominant follicles grow to about 20–25 mm and then rupture spontaneously at ovulation. After ovulation, it becomes the corpus luteum (CL) and undergoes cellular hypertrophy and increased vascularity. A corpus luteum is seen on ultrasound as a thick-walled cyst often with irregular margins and low-resistance flow around it on Doppler. The irregular inner margins of a corpus luteum often give it a classic ‘crenated appearance’. The contents of the corpus luteum usually show turbid fluid with internal echoes. A CL is usually less than 3 cm but may occasionally be larger and may appear complex showing clots within.


Fig. 7.17
Ovaries of women in the reproductive age group. (a) The ovary shows a developing follicle (in the proliferative phase of the menstrual cycle). (b) The ovary shows a corpus luteum (in the secretory phase of the menstrual cycle). (c) Specimen of a corpus luteum from an excised ovary
Postmenopausal Ovaries (Fig. 7.18)
Normal postmenopausal ovaries are smaller in size (1.2–5.8 cc), less echoic and show fewer follicles than in premenopausal women. Because of their small size and the absence of follicles, they are often difficult to locate, both on TAS and TVS. Small punctate echogenic foci are often seen in postmenopausal ovaries, which helps in identifying the ovaries in postmenopausal women. The ovarian size correlates with the duration of menopause. A volume of more than 8 cc or an ovary that is twice as large as the contralateral ovary is considered abnormal. Simple ovarian cysts may be seen in postmenopausal women, particularly in early menopause. In late menopause also, a smaller cyst (less than 1 cm) may be seen andshould be reported if noted.


Fig. 7.18
Postmenopausal ovary. (a) Shows small ovarian size. (b) Ovaries show no follicle. (c) Small punctate echogenic focus (arrows) seen in the ovary, which if present is often very useful while searching for the ovaries during TVS, in postmenopausal women
7.3 Polycystic Ovaries (PCO)
Polycystic ovarian syndrome (PCOS) is the commonest endocrinal abnormality seen in about 6.6 % in women in the reproductive age group. This syndrome carries significant health risk, including diabetes, cardiovascular disease, endometrial hyperplasia and infertility. Most of these women present with infertility, amenorrhoea or oligomenorrhoea, menorrhagia, obesity and hirsutism. Endometrial hyperplasia and, occasionally, endometrial carcinoma may be seen in these women, secondary to long-term exposure to unopposed oestrogen.
There is no universal consensus on diagnostic criteria. In 1935, Stein and Leventhal first described a condition consisting of amenorrhoea, obesity and masculinising features, now known as polycystic ovarian syndrome. The Rotterdam PCOS consensus group brought out criteria in 2003 for the definition of PCOS. The Rotterdam criteria require at least two of the three conditions given below to be present for the diagnosis of PCOS:
- 1.
Anovulation or oligoovulation.
- 2.
Clinical or biochemical signs of hyperandrogenism.
- 3.
Polycystic ovaries on ultrasound – The Rotterdam criteria for polycystic ovaries on ultrasound are more than 12 or more follicles measuring 2–9 mm and/or an ovarian volume more than 10 cc in one or both ovaries. Later it was believed that instead of the criterion of more than 12 follicles, a criterion of more than 19 follicles was more appropriate. The new PCO criterion proposed (HR 2013), however, is an antral follicle count of 25 or more. Here, it is assumed that the evaluation is done by a transvaginal scan that can provide good resolution. Rotterdam criteria, however, remain at more than 12 follicles, probably because TVS is not routinely used the world over.
Evaluation of the ovaries for the diagnosis of polycystic ovary should ideally be done between day 2 and day 4 of the menstrual cycle by a transvaginal scan (if possible). Patient should not be or recently have been on hormonal medication.
Antral follicle count (AFC) is increased: The presence of antral follicles (2–9 mm) in one or both ovaries. The number of antral follicles for diagnosis will vary depending on what criteria are used in each institution. Rotterdam criteria are an AFC of more than 12. However, most centres now consider an AFC of 25 or more for diagnosing PCO.
Ovarian volume is increased: An ovarian volume of more than 10 cc is used for the diagnosis of PCO (in the absence of a dominant follicle or a corpus luteum).
Peripherally arranged antral follicles (‘pearl necklace appearance’) are seen in classic polycystic ovaries. However, it is not a requisite for the diagnosis of PCO based on Rotterdam criteria.
Abundant stroma is a feature of classical polycystic ovaries which is seen centrally. It is generally more hyperechoic than usual. This stroma generally shows increased vascularity.
3D ultrasound with volume analysis is being increasingly used for the diagnosis of PCO and for the evaluation of PCO in patients undergoing ovulation induction. This helps to enhance the accuracy, speed and ease of measuring AFC. Ovarian volume can also be calculated with 3D volume studies.
The endometrium in patients with polycystic ovaries may be thickened, heterogeneous, with tiny cysts and show increased vascularity. This could be secondary to endometrial hyperplasia or, rarely, endometrial carcinoma.

Fig. 7.19
(a) Case 1 – A classic case of polycystic ovaries with peripherally arranged antral follicles (‘pearl necklace appearance’), central hyperechoic, bulky stroma and increased ovarian volume. (b, c) Case 2 – Polycystic ovaries showing increased antral follicle count. Ovarian volume is also increased. (d) Thickened endometrium showing small cystic spaces suggestive of cystic hyperplasia in Case 2

Fig. 7.20
SonoAVC software is useful in obtaining an antral follicle count. This is particularly useful in the treatment of women with infertility. It provides an accurate and quick way of counting antral follicles
7.3.1 PCO in the Absence of PCOS
The presence of polycystic ovaries (PCO) is seen in about 23 % of women of reproductive age group; however, only some of these have PCOS (i.e. associated anovulation and hirsutism). The significance of PCO in the absence of the syndrome primarily lies in the fact that these women are more likely to hyperstimulate with gonadotrophins during ovulation induction. Since a large number of women with polycystic ovaries on ultrasound do not have polycystic ovarian syndrome (which is a disease associated with a lot of other medical issues), it may be prudent to communicate this in the ultrasound report, stating that though the ovaries appear polycystic, it does not imply diagnosis of polycystic ovarian syndrome, which requires other features of hyperandrogenism and/or ovulatory dysfunction.
7.4 Ovarian Masses
Ovarian masses can be broadly classified into functional cysts, endometriomas and neoplastic masses.
Other causes of ovarian enlargement are masses of inflammatory origin (discussed in Chap. 9 in the section on PID), hyperstimulation (discussed in Chap. 13), ovarian torsion (discussed in Chap. 11) and ovarian ectopic (discussed in Chap. 10 in the section on ectopic pregnancies).
The differential diagnosis for ovarian masses is dealt with in Chap. 14 in the section on adnexal masses.
7.4.1 Functional or Physiological Cysts (Figs. 7.21, 7.22 and 7.23)
All functional cysts of the ovary are unilocular and are generally less than 8 cm. They are usually unilateral (unless the patient is undergoing ovulation induction). Any fluid containing mass in the ovary is a cyst; therefore, all follicles are cysts. One must, however, avoid the term cyst for a regular ovarian follicle because for most patients, the term cyst has a pathological connotation. Follicles that have a diameter of more than 3 cm can be called a follicular cyst. Follicular cysts are generally asymptomatic.
A corpus luteal cyst is usually thick walled and seen in the secretory phase of the menstrual cycle. Corpus luteal cysts may be symptomatic and occasionally may cause acute pain and haemoperitoneum due to haemorrhage from a corpus luteal cyst. In pregnancy, corpus luteal cysts may be larger and persist for a longer period. Their size reaches a maximum at about 10 weeks, and they generally regress by about 16 weeks of pregnancy. Corpus luteal cysts are generally haemorrhagic cysts but could be simple clear cysts.



Fig. 7.21
Physiological cyst. (a) Follicular cyst. (b) Corpus luteal cyst (212 ml) in a pregnant lady. (c) CL cyst in ‘b’ has regressed completely following pregnancy

Fig. 7.22
Corpus luteal cyst. (a) At early pregnancy scan, the cyst was large. (b) At NT scan, the cyst had regressed
A haemorrhagic cyst is most often a corpus luteal cyst, but it could also be a follicular cyst with haemorrhage. An endometrioma with recent haemorrhage can also show features of a haemorrhagic cyst.
Ultrasound Features of a Haemorrhagic Cyst (Fig. 7.23)
Unilocular
Generally thick walled
It shows internal echoes which may be hyperechoic (when haemorrhage is recent) or reticular, giving it a ‘cobweb’ appearance, or it may show diffuse low-grade internal echoes (especially when it is regressing).
A clot within a haemorrhagic cyst may retract away from the cyst wall and the demarcation is often clearly seen. Clots usually have concave margins facing the lumen of the cyst. The entire margin of the clot facing the cyst wall may not be adherent to the cyst wall, and part of it may be seen some distance away from the cyst wall. The clot, in addition, may show jelly-like movements on intermittent pressure by the TVS probe. Most importantly, a clot will not show any flow within it on Doppler because it is avascular. This is a very important feature to help differentiate it from solid tissue within a cyst, which is generally of great concern.
The appearance of a haemorrhagic cyst varies with time as the haemorrhage within gradually resolves.


Fig. 7.23
Haemorrhagic cysts vary in appearance. (a) Hyperechoic areas within. (b) Hypoechoic with few internal echoes. (c) Reticular appearance of the clot within the cyst. Clot retracting away from the cyst wall (arrow). (d, e) Clot within the cyst shows concave margins (characteristic feature). (f, g) Haemorrhagic cyst does not show flow within on Doppler, which helps differentiate it from masses with solid tissue within
Summary: Functional Cysts
History and Symptoms
Women are of the reproductive age group.
It is important to correlate the findings with the phase of the menstrual cycle and pregnancy status.
Previous reports and images are also useful for correlation.
On ultrasound, these cysts are unilocular, clear or haemorrhagic (complex echoes) and generally less than 8 cm.
Follow-up is useful when in doubt. On rescan after 2–3 months, the cyst will either show complete regression or would have changed in size and appearance.
7.4.2 Endometriotic Cysts (Endometriomas)
Endometrial tissue is seen within the ovary in cases with endometriomas. Endometriotic cysts are also called ‘chocolate’ cysts because of the thick chocolate-coloured fluid within these cysts. Further information on endometriosis is available in Chap. 8.
Ultrasound Features of Ovarian Endometriomas (Figs. 7.24, 7.25, 7.26, 7.27, 7.28, 7.29, 7.30 and 7.31)
Typically they appear as well-defined hypoechoic cysts with uniform low-grade internal echoes, which gives them a ‘ground glass’ appearance.
Not all ‘ground glass’ cysts are, however, endometriotic. In premenopausal women, the majority are endometriotic, a few are malignant and the rest are benign. However, in postmenopausal women, the majority are malignant and the rest are endometriotic or benign.

Holsbeke et al. UOG 2010
Cysts with thick turbid fluid can sometimes give the appearance of being solid masses. They can be differentiated from each other (as elucidated in Chap. 1).

Fig. 7.24
Characteristic ‘ground glass’ appearance of endometriotic cysts. (a) On TAS, (b) on TVS
Endometriotic cysts are often multiple and bilateral.
The multiple cysts are often closely packed, giving them a multilocular appearance and making it difficult to outline them at times. In such cases, the number of cysts can be identified as follows:
- 1.
The walls of the cysts can be visualised as bright lines or darker linear shadows.
- 2.
Doppler flow is often seen in the cyst walls/ovarian tissue intervening between the cysts.
- 3.
Density of echoes may differ in each cyst.
Sometimes, the cysts within an ovary are seen communicating with each other due to disruption of the intervening tissue. This is more often seen following conservative surgery for endometriosis.

Fig. 7.25
Multiple endometriotic cysts giving the ovary a multilocular appearance. Features that help delineate and count these cysts are (a) hyperechoic linear compressed ovarian tissue between cysts (arrow), (b) dark linear shadow between two cysts (arrow), (c) flow in the compressed ovarian tissue between cysts (arrow), (d) varying echodensities of the cysts
- 1.
Cyst walls
Typically the cyst walls are thick and regular.
At times, wall nodularity may be noticed because of hyperechoic, irregular areas along the inner cyst walls which are nothing but clot and debris secondary to the haemorrhage within.
Small hyperechoic foci are often seen in the cyst walls of these endometriotic cysts. They are believed to be cholesterol deposits in the cyst walls and are seen in about 35 % of endometriomas. As an isolated marker for endometriomas, they have the highest LR of 6.2. The LR increases to 32 if these hyperechoic foci are seen in a ‘ground glass’ cyst. An association between hyperechoic foci in the cyst walls and endometriomas is well established. Hyperechoic foci in sonographically normal ovaries are not, however, predictive of endometriosis.
On Doppler, the cyst walls show minimal high-resistance flow with colour score of 2–3. Increased flow may be seen in pregnancy or with secondary infection.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


