(1)
Department of Fetal Medicine and Obstetric & Gynecological Ultrasound, Manipal Hospital, Bangalore, Karnataka, India
The myometrium is the muscular tissue of the uterus and the cervix, which encloses the uterine cavity and its lining, the endometrium. The myometrium is generally isoechoic (similar to the liver) and homogeneous. The myometrial echogenicity, thickness, contour and presence of any mass or cysts are noted during ultrasound examination. The two commonly encountered pathologies of the myometrium are fibroids and adenomyosis.
3.1 Evaluation of Myometrium
The myometrium can be evaluated both on transabdominal and transvaginal scan. Ideally, like in all other conditions, both TAS and TVS should be done, as they complement each other. In myometrial pathologies like fibroids and adenomyosis, the uterus is sometimes of large size extending beyond the pelvis, and a TAS becomes essential to evaluate the uterus. In addition, TVS may be suboptimal if there is a large mass (like a fibroid) in the cervix or lower corpus that causes shadowing and prevents assessment of the structures above it. When the uterus is of a large size, one may not require a very full bladder because the large uterus itself often pushes the bowels away into the upper abdomen.
The evaluation of the uterus should be systematic, and reporting should be standardised. The MUSA (Morphological Uterus Sonographic Assessment) statement is a consensus statement by a panel of experts (MUSA consensus group) for terms, definitions and measurements for describing and reporting the myometrium and its pathologies. The terms and definitions laid down by the MUSA will be relied on for the most part in this chapter.
The uterus is first evaluated in the sagittal and transverse sections on 2D. 3D is used to get a coronal section of the uterus, whichprovides good information of the external uterine contour, cavity shape, endomyometrial junction and relation of myometrial pathology to the endometrial mucosa and serosa. Dopplers are used as and when relevant, typically when pathology is noted.
Uterine size: With myometrial pathologies, the uterus is often enlarged. It could be asymmetrically enlarged or globally enlarged. The uterus is measured in three dimensions:
The total length of the uterus is the length from the upper margin of the uterine fundus to the external os in the sagittal section of the uterus.
The anteroposterior diameter of the uterus is the maximum AP measurement in the sagittal section of the uterus.
The transverse diameter of the uterus is the maximum transverse measurement taken in the transverse section of the uterus.
The thickness of the anterior and posterior myometrial walls is measured from the external uterine serosa to the endometrial margins and should include the JZ. The measurement is done in the sagittal plane, perpendicular to the endometrium, in a single image where the endometrium is thickest. The myometrial walls are not measured routinely, unless there is some pathology or asymmetry noted.
Any mass, like a fibroid, seen within the myometrium is measured in three orthogonal dimensions (three measurements perpendicular to each other), in two images whose plane is perpendicular to each other (as explained in Chap. 2).

Fig. 3.1
Uterus measured on TAS. (a) LS and (b) TS views

Fig. 3.2
Thickness of asymmetrical myometrial walls measured in a midsagittal section, perpendicular to the endometrium. The anterior wall is much thicker than the posterior wall

Fig. 3.3
Myometrial fibroid measured in three dimensions in two perpendicular planes
Qualitative Assessment of the Myometrium (Fig. 3.4)
The outer serosal contour of the uterus is noted to see if it is regular or not.
The uterine myometrial echogenicity is assessed as uniform (homogeneous) or non-uniform (heterogeneous).
The normal myometrium is used as a standard to evaluate echogenicity of other structures in the myometrium, i.e.,structures that are as echogenic as the normal myometrium are considered to be isoechoic. Lesions that are less echogenic are termed hypoechoic, whereas those which are more echogenic aretermed hyperechoic.
The symmetry of the myometrial walls is then assessed. Asymmetrical thickening of the myometrium is often due to pathology. If the walls are of symmetric thickness, then one does not need to measure them.
The myometrium is also assessed for the presence of any mass or cyst. These are further assessed for their echogenicity, blood flows, acoustic shadowing and relation to the endometrial mucosa and outer serosa.
In order to assess the location of the pathology in the myometrium, it is important to delineate the endometrial cavity which could be a challenge when there are existing myometrial pathologies like fibroids. One useful tip that may help to trace the endometrium is to find the cervical canal and trace it upwards.

Fig. 3.4
Contour of uterus in two different cases. (a) Regular, in a normal uterus. (b) Irregular in a uterus with multiple fibroids (F), which are seen as circumscribed hypoechoic masses with shadowing
Assessing the ‘Junctional Zone’ (JZ) (Fig. 3.5)
The junctional zone is basically the inner myometrium composed of longitudinal and circular closely packed smooth muscle fibres. The JZ is visualised as a hypoechoic (dark), halo (layer) just beside the endometrium. It is clearly visualised on 3D rendered images and VCI (discussed in Chap. 2).
The JZ may be well defined or poorly defined.
It may be regular, irregular or interrupted.
It may not be possible to assess the junctional zone in a mid-positioned uterus or shadowing by fibroids.
In adenomyosis (discussed later in a separate section), the JZ shows tiny cystic spaces, hyperechogenic dots, hyperechoic buds or echogenic lines. It may also be thickened in cases of adenomyosis.


Fig. 3.5
Endomyometrial junction (EMJ). (a) Diagrammatic representation. (b, c) Regular EMJ on 2D and 3D. (d, e) Irregular EMJ on 2D and 3D in a patient with adenomyosis
The arcuate vessels can be seen in the outer part of the myometrium running parallel to the serosa. Perpendicular to the arcuate vessels are the radial arteries and veins. Flow can be seen in these arcuate and radial vessels of the myometrium. Power Doppler is preferred to colour Doppler in assessing flows in the myometrium because power Doppler is more sensitive to detect flows in small vessels and low-velocity flows.
Whenever a lesion is visualised, its vascularity should be reported using subjective colour scoring from 1 to 4 (discussed in detail in Chap. 2 under the section on Doppler). One can also do a spectral flow analysis to assess resistance to flow (RI or PI) and flow velocity (PSV) in a vessel.
Lesions of the myometrium may show circumferential flow running along the periphery of the lesion or intralesional flow running through the mass.

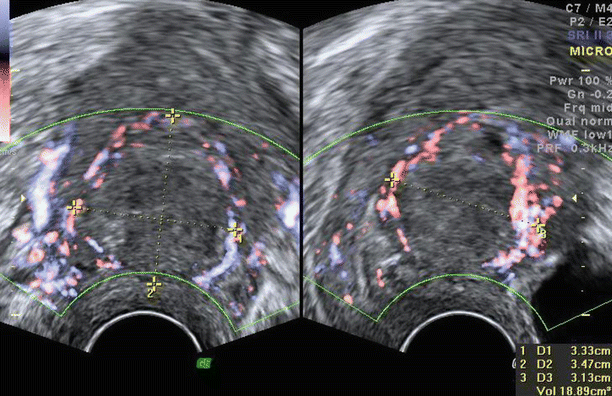

Fig. 3.6
Normal vasculature of myometrium on (a) colour Doppler and (b) on 3D glass body display. Short thick arrows showing arcuate vessels, medium-sized arrows showing radial vessels and long arrows showing spiral vessels

Fig. 3.7
Peripheral flow seen on Doppler around a fibroid
3.2 Normal Myometrium (Figs. 3.8 , 3.9 and 3.10 )
Uterine size, myometrial thickness and the proportion of the uterine body to the cervix change with the age of an individual and occurrence of pregnancy.
Neonatal uterus:
The uterus is relatively prominent because of exposure to maternal hormones with an average uterine length of 3.5 cm and thickness of 1.4 cm.
Paediatric uterus (Fig. 3.8):
The uterus in the paediatric age group is usually less than 3 cm and becomes 3–4.5 cm in the prepubertal age group. At puberty the uterus is usually 5–8 cm long. The cervix in the paediatric age group is prominent and equal in proportion to the uterine body. At puberty, the uterine body becomes thicker and pear shaped with a uterine body to cervix ratio of about 1.5: 1.
Uterus in the reproductive age group (Fig. 3.9):
The size of the normal uterus varies with parity. Dimensions of a normal uterus are about 8 cm in length, 4 cm in AP diameter and 5 cm in width,with the multiparous uterus being about a centimetre larger in each dimension. The uterine body is approximately twice the size of the cervix. After delivery, the uterus undergoes physiological evolution during the 6–8 weeks of puerperium to return to its normal size. Immediately after delivery, the uterus is about 20 cm in length, and after 3 weeks, it is about 11 cm in length.
Postmenopausal uterus (Fig. 3.10):
The uterus is smaller in size, usually less than 7.5 cm. This of course depends on the time since menopause, parity of the patient and the presence of pre-existing myometrial pathology. The uterine body to cervix ratio approaches 1:1. In elderly postmenopausal women (particularly those with vascular disease, diabetes or hypertension), calcified arcuate vessels are noted. These are seen in the outer myometrium as peripheral bright scattered foci with some shadowing which are arranged circumferentially around the uterus.




Fig. 3.8
Prepubertal uterus (a) measuring 3.5 × 0.9 × 1.2 cm. (b) Cervix appears relatively bulkier than the uterus

Fig. 3.9
Uterus in a patient of the reproductive age group showing normal homogeneous myometrium

Fig. 3.10
Postmenopausal uterus with calcified arcuate vessels seen as hyperechoic scattered foci close to the uterine periphery (arrows), on (a) LS and (b) TS of the uterus
3.3 Fibroids (Leiomyoma or Myoma)
Fibroids, also known as leiomyomas, are benign tumours of the myometrium. They are composed of smooth muscle cells and connective tissue in densely packed whorls. They are common, and about 40 % of women by the age of 40 years have fibroids. Very often they are multiple.
Many of the women with fibroids are asymptomatic, while others may have symptoms like menorrhagia, polymenorrhagia, intermenstrual bleeding, dysmenorrhoea and subfertility. With submucous fibroids and fibroid polyps, intermenstrual bleeding (metrorrhagia) could be a presenting complaint.
Fibroids typically appear as round, well-defined, oval or lobulated solid masses seen in the uterus or arising from it.
Fibroids show variable echogenicity depending upon the proportion of muscle cells and fibrous stroma and the presence of any degenerative changes. They can appear from hypoechoic to hyperechoic.
Fibroids generally show linear stripy fan-shaped internal acoustic shadowing and also shadowing from its edges (reported as ‘edge shadows’).
They may show calcification and cavitation.
On Doppler, fibroids typically show pericapsular flow (i.e. circumferential flow around its margins). Some amount of intralesional flow is also commonly seen within the fibroid. Some fibroids may, however, show high vascularity (increased vascularity seems to be related to increased cellularity in fibroids).
Despite the variable appearance of fibroids, diagnosing them on ultrasound is not challenging because any mass in the uterus (once an adenomyoma is excluded) or arising from it is almost always a fibroid.








Fig. 3.11
Typical fibroids (a, b) are seen on ultrasound as well-demarcated, solid masses with stripy, linear, fan-shaped, internal shadowing and shadowing from their edges (arrows)

Fig. 3.12
Fibroids showing varying echogenicity. (a) Hypoechoic, (b) isoechoic (arrow) and (c) hyperechoic

Fig. 3.13
Fibroids (a, b) showing complex internal echoes

Fig. 3.14
Fibroids (a, b) showing cavitation (seen as irregular, anechoic or hypoechoic, cystic areas within the fibroid)

Fig. 3.15
Fibroids showing calcification. (a) Peripheral calcification, appearing like a shell around the fibroid. (b) Internal calcification causing acoustic shadowing

Fig. 3.16
Vascularity of fibroids. (a, b) Fibroids showing typical peripheral vascularity. (c) Atypical fibroid with significant internal vascularity. (d) 3D power Doppler with glass body display showing the vascular morphology in fibroids with high vascularity

Fig. 3.17
Fibroid. (a) Isoechoic fibroid seen in the anterior wall of uterus with its margins not clearly defined. (b) Peripheral vascularity helped define the margins of the fibroid and confirm the diagnosis
3.3.1 Fibroid Mapping
3.3.1.1 Basics of Fibroid Mapping (Figs. 3.18, 3.19 and 3.20)
To map or describe the location of any mass like a fibroid, in the uterus, one must know four parameters:
- 1.
How superior or inferior the mass is. For this, the uterus can be divided into fundus, upper corpus, midcorpus, lower corpus and cervix.
- 2.
How anterior or posterior the mass is. The mass could be in the anterior wall or the posterior wall.
- 3.
How much to the left or right the mass is. The mass could be right sided, left sided or in the midline.
- 4.
How close is the mass to the inner endometrial mucosa or the outer serosa? The mass could be subserous, intramural or submucous. A fibroid or mass reaching both the serosa and the mucosa is termed transmural. In some cases, noting the exact distance of a fibroid from the mucosa or serosa provides useful information to the surgeon in planning surgical management. This is particularly so in cases of submucous fibroids whichare being considered for hysteroscopic resection, where the thickness of the remaining overlying myometrium becomes important.
At times, the uterine architecture is significantly distorted by multiple fibroids making their location within the uterine body difficult to ascertain. To locate them, finding the endometrial stripe is important. If this becomes difficult due to shadowing by multiple fibroids, tracing it up from the cervical canal is usually helpful.
Fibroids can be located and mapped with the help of a 2D live scan, by gently moving the probe from the left to the right in a longitudinal section and from up to down in a transverse section. Visualisation of the fibroid in a coronal section is better on 3D, particularly in assessing the relation of the fibroid to the endometrial cavity.
Locating the fibroid or ‘fibroid mapping’ is very important because:
It gives us information about whether the fibroid is the cause for the patient’s symptoms. For example, fibroids that are submucous are more likely to cause menorrhagia; fibroid polyps are more likely to cause metrorrhagia and dysmenorrhoea.
It also helps to decide if surgery is required and, if so, the type of surgery that is appropriate – a hysterectomy, myomectomy or a hysteroscopic resection.
A good mapping also helps the surgeon during surgery to know the exact site of the various fibroids for a proper surgical approach and to ensure that all the fibroids are managed optimally.
Submucous fibroids have again been graded into:
G0 – completely intracavitary
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


