CHAPTER 6 TUMORS AND TUMOR-LIKE LESIONS
PEDIATRIC ‘BLASTOMAS’ / ‘SMALL ROUND CELL’ TUMORS
INTRODUCTION
• A large number of pediatric embryonal tumors share a component with a ‘small round blue cell’ phenotype (SRBCT)
• Many of the diagnostic morphological findings require assessment of overall architecture and other features which may not necessarily be present in needle core biopsy specimens
• Immunohistochemical staining and molecular techniques may be required in a large proportion of such specimens for definitive diagnosis
• A targeted immunohistochemical panel should be performed to determine appropriate positive and negative findings
• Since morphological features may be non-diagnostic, adequate clinical history, imaging and results of other investigations are essential for appropriate interpretation
• The most immediate issues for management in most cases are the differential between leukemia / lymphoma, rhabdomyosarcoma, other high grade embryonal sarcoma such as PNET and other less aggressive tumors
HANDLING OF PEDIATRIC TUMOR SPECIMENS
• Many pediatric tumors require additional investigations for their definitive diagnosis and prognosis
• All diagnostic pediatric tumor specimens should be submitted to the laboratory as fresh tissue immediately following biopsy where possible
• The specimen should be divided according to predetermined standard operating procedures, with aliquots for:
• All laboratories providing a diagnostic pathology service for pediatric tumors should have facilities available for handling of fresh specimens and access to appropriate molecular diagnostic services
• Advances in technical aspects now mean that many molecular techniques, such as FISH and RT-PCR, may also be performed on routinely fixed paraffin embedded material
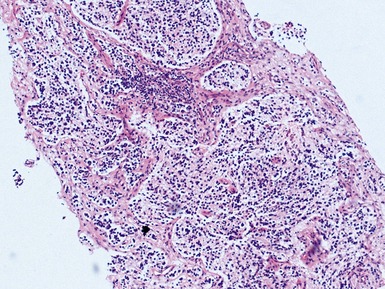
Issues specific to needle core biopsy specimen interpretation in pediatric tumor diagnosis (Figs 6.1, 6.2)
• In an increasing number of centers, primary diagnostic investigation of suspected pediatric tumors is on the basis of an image guided needle core biopsy
• A full range of ancillary investigations is possible in the vast majority of needle biopsy specimens in centers experienced in their handling
• Limited material is available for morphological analysis in such cases
 many of the ‘diagnostic’ architectural features referred to in standard texts may not be applicable to the interpretation of these limited samples
many of the ‘diagnostic’ architectural features referred to in standard texts may not be applicable to the interpretation of these limited samples
 many of the ‘diagnostic’ architectural features referred to in standard texts may not be applicable to the interpretation of these limited samples
many of the ‘diagnostic’ architectural features referred to in standard texts may not be applicable to the interpretation of these limited samples• ‘Molecular profiling’ of tumors is likely to become of increasing importance for both determining diagnosis, and prognosis and management in pediatric tumors
• Needle biopsies are safe, with a high adequacy rate and diagnostic yield and associated low morbidity
PERIPHERAL NEUROBLASTIC TUMORS (NTS)
• Classified on the basis of the histological features into four main types [International Neuroblastoma Pathology Classification (INPC) system; Shimada et al 1999]:
NEUROBLASTOMA
• Age, clinical stage, molecular findings and undifferentiated subtype influence overall survival (Lastowska et al 2002, Shimada et al 2001, Goto et al 2001, Ikeda et al 2002, Burgues et al 2006, Navarro et al 2006, Sano et al 2006)
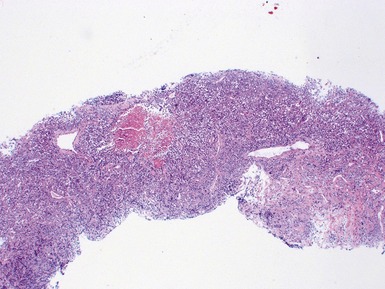
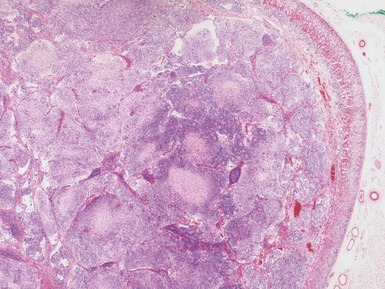
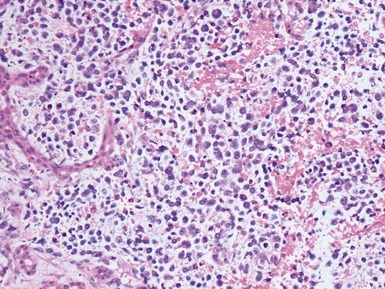
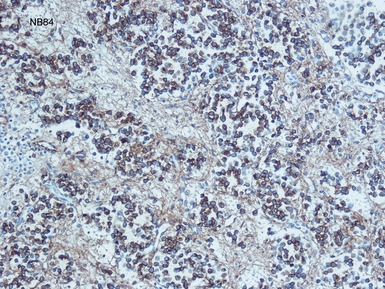
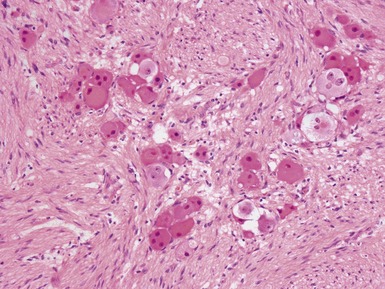
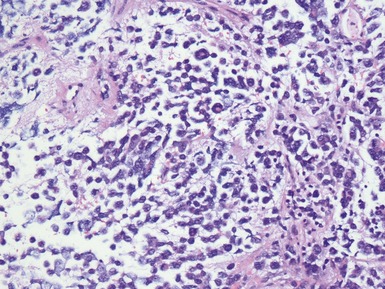
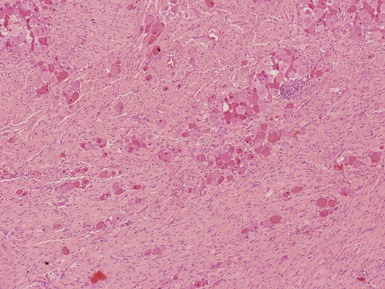
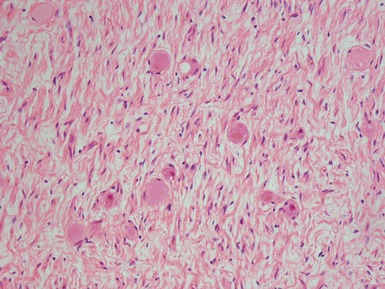
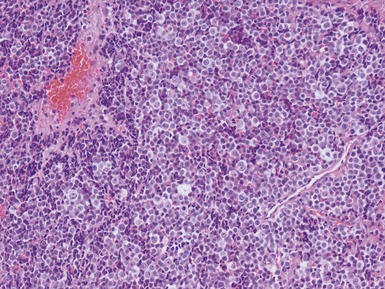
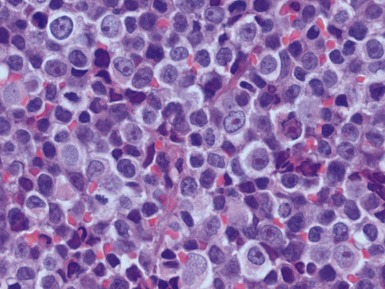
Histopathological features (Figs 6.3–6.8)
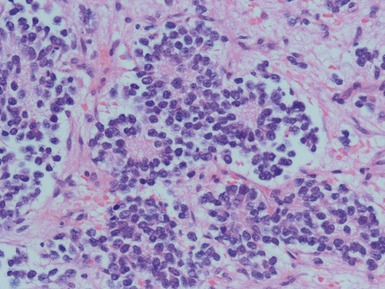
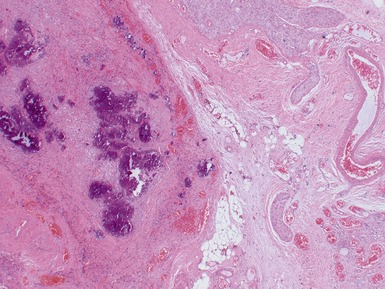
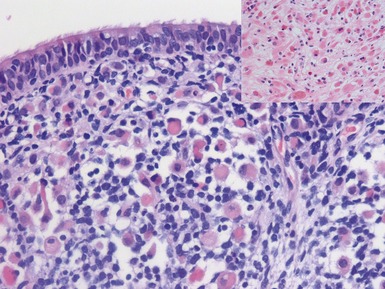
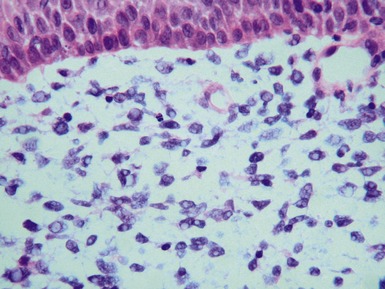
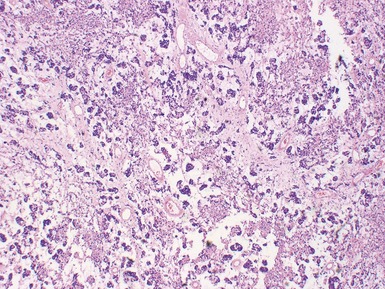
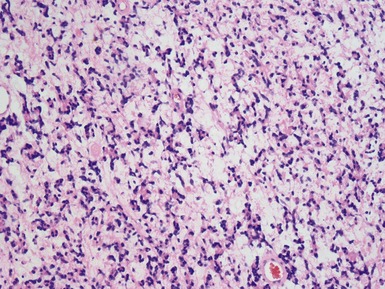
Figs 6.5–6.8 Photomicrographs of cases of neuroblastoma, demonstrating tumor composed of sheets and nests of small ovoid cells within a variably prominent neurofibrillary background.
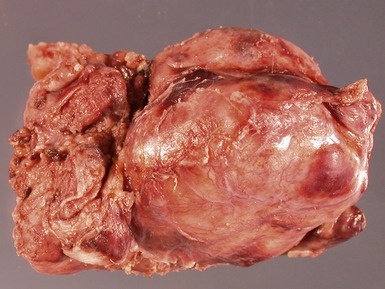
GANGLIONEUROBLASTOMA, INTERMIXED (Figs 6.11–6.14)
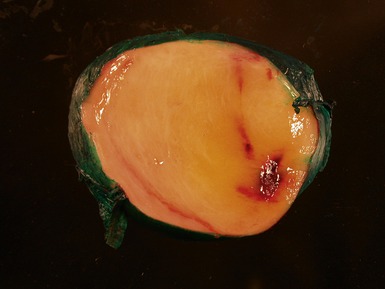
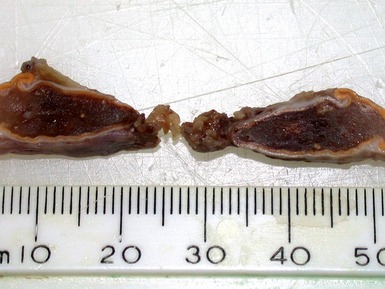
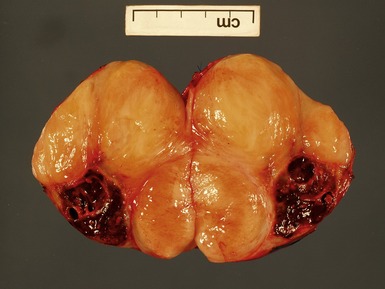
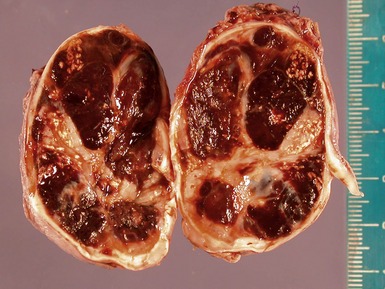
Figs 6.11–6.12 Macroscopic photographs of ganglioneuroblastoma, intermixed, demonstrating solid expansile masses with yellow cut surface. Note the area of hemorrhage in Fig 6.12 compared to the area of neuroblastomatous tissue in ganglioneuroblastoma, nodular in Fig 6.15.
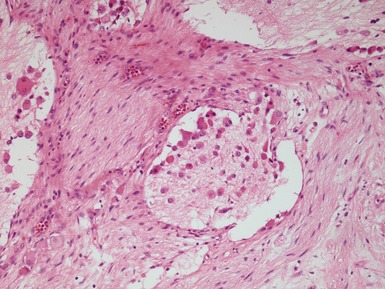
GANGLIONEUROBLASTOMA, NODULAR (Figs 6.15–6.17)
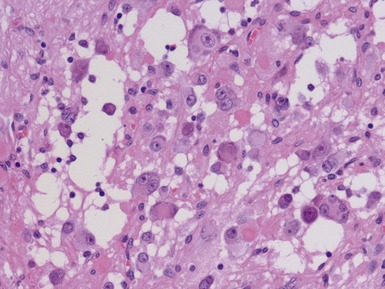
NEUROBLASTOMA (SCHWANNIAN STROMA POOR), NOS
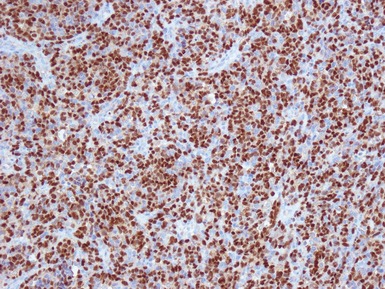
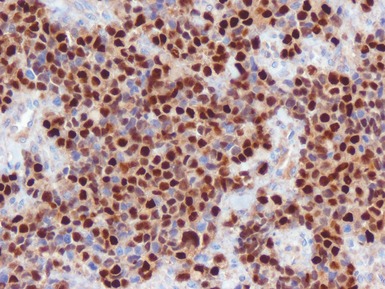
Additional investigations
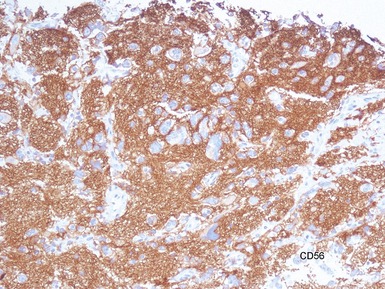
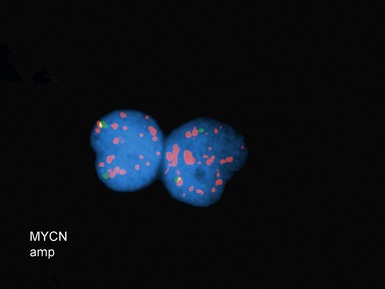
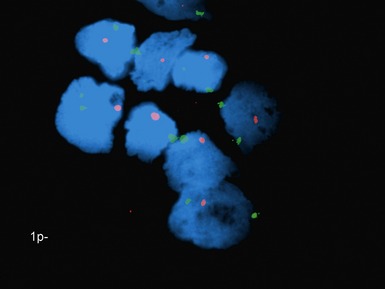
• Molecular studies are mandatory in risk stratification and management planning in neuroblastic tumors
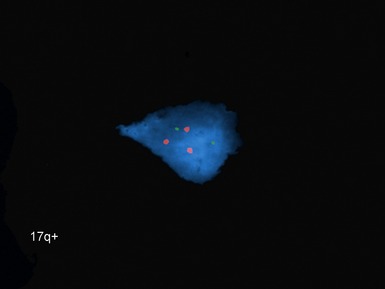
• Current biological markers associated with adverse prognosis (Lastowska et al 2001, Schwab et al 2003) (Figs 6.21–6.23):
EMBRYONAL RHABDOMYOSARCOMA
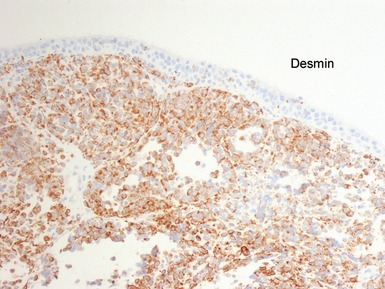
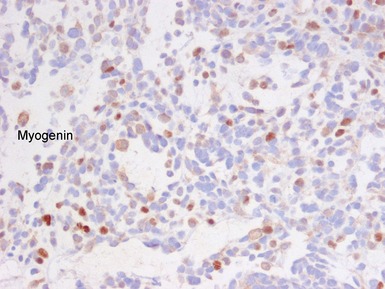
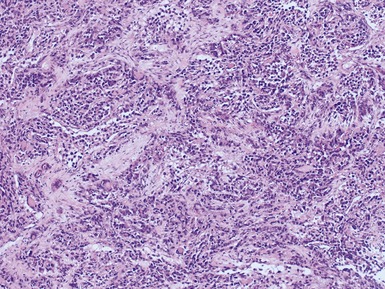
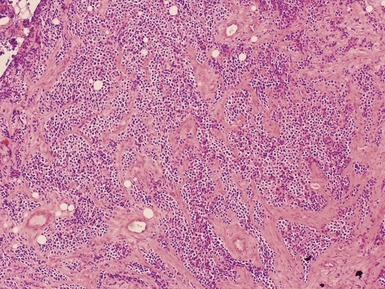
Histopathological features (Figs 6.26–6.31)
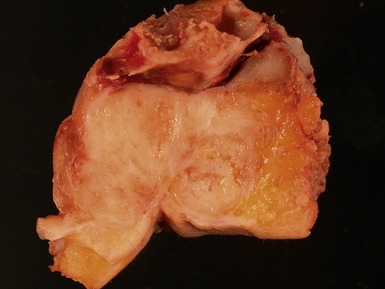
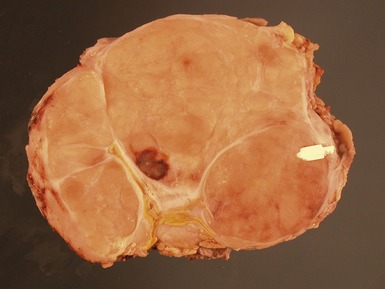
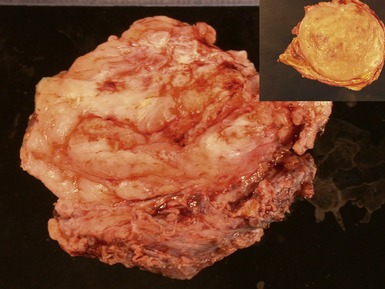
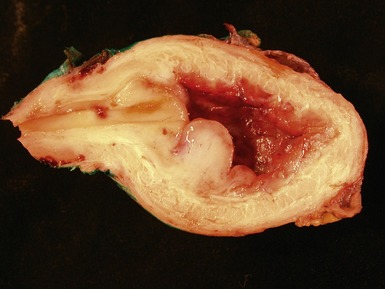
Figs 6.26–6.27 Macroscopic photographs of bladder and soft-tissue rhabdomyosarcomas, demonstrating poorly circumscribed, infiltrating lesions of viable cream-colored tumor.
Differential diagnosis and pitfalls
• Marked pleomorphism may be a feature of a subgroup of eRMS in childhood with more aggressive course
ALVEOLAR RHABDOMYOSARCOMA
Differential diagnoses and pitfalls
• Since the introduction of routine molecular testing the relative prevalence of morphological spectrum of ARMS has expanded
• Some cases previously thought to be eRMS on morphological grounds found to have translocation of ARMS since introduction of routine molecular testing
EXTRASKELETAL MYXOID CHONDROSARCOMA
Histopathological features