Agent
Onset of action (min)
Duration of effect (min)
Maximum total dose (mg)
Lidocaine
2–5
30–120
300
Bupivacaine
5–10
120–240
175
Ropivacaine
3–15
120–360
200
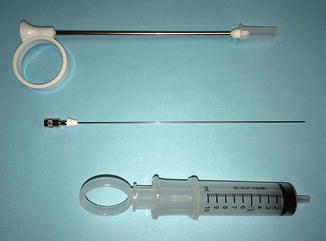
Fig. 14.1
Iowa trumpet needle guide, 20-gauge spinal needle with spacer, and 10 cc syringe
Trigger point injections should be performed using sterile technique. When injecting myofascial trigger points present in the levator ani, the patient should be placed in the dorsal lithotomy position, prepped, and draped. A vaginal exam is performed to confirm the location of the trigger points and the planned site(s) of injection. We tend to first perform a bilateral pudendal nerve block just medial to the ischial spines to achieve analgesia before administering trigger point injections throughout the levator ani. Injections can be performed transvaginally, transperineally, or subgluteally. In the latter approach, the needle is inserted inferior and medial to the ischial tuberosities and is guided towards a finger placed transvaginally or transrectally [5]. Transperineal injections can be performed for trigger points located in the perineal muscles or puborectalis, while the transvaginal approach offers access to trigger points located in the obturator internus muscles. The authors prefer either a transperineal or transvaginal approach (Fig. 14.2) given the proximity of the injection site to the trigger point and the relative ease of injection.
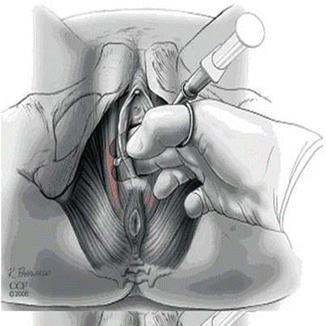

Fig. 14.2
Trigger point injection using a transvaginal approach. From Langford CF, Udvari NS, Ghoniem GM. Levator ani trigger point injections: an underutilized treatment for chronic pelvic pain. Neurourol Urodyn 2007;26(1):59-62
After identifying the location of the trigger points, the needle is inserted which may elicit a local twitch response [4, 5]. The syringe should be aspirated to avoid intravascular injection. Local anesthetic is then injected into the trigger point. We use 1 % lidocaine initially, and if a therapeutic effect is achieved, we then inject 9 cc of 0.5 % bupivacaine mixed with 1 cc of 40 mg of triamcinolone for longer lasting pain relief at a subsequent visit. Injections may be accomplished while slowly withdrawing the needle [4]. The needle may also need to be redirected to adequately infiltrate the entire trigger point. The number of injections performed depends on how many trigger points are identified on exam. Although we administer a steroid agent in addition to a local anesthetic, some authors [5] caution against use of steroid agents for myofascial trigger point injections because of the theoretical concern of muscle wasting with repeated use.
Botulinum neurotoxin has also been used for the treatment of trigger points. It is administered using the same technique of injection employed for injection of local anesthetic and steroid agents. The typical dose of botulinum toxin varies in the literature and most of the evidence supporting its efficacy is derived from treatment of conditions involving generalized levator ani spasm [6, 10–15]. Doses between 20 and 400 units diluted in sterile normal saline have been used [15]. The clinical effects of toxin injection may not become apparent until 24 to 72 h post-procedure [11] and have been reported to last between 2 and 6 months [10, 11]. Because botulinum toxin is a heat-labile protein, it may be prudent to instruct patients to avoid hot baths, Jacuzzis, steam rooms, and saunas for the first 2 weeks following injection [5].
Complications of Trigger Point Injections
Prior to the procedure clinicians must confirm the patient does not have an allergy to the agent that will be injected. They must also be aware of the possible risks of injection including infection, hematoma formation, and the potential for syncopal or presyncopal episodes. Toxicity from local anesthetic use can occur with intravascular injection or by exceeding the maximum recommended dosage. Potential cardiovascular effects that may occur include cardiac arrhythmias and cardiac arrest. Symptoms of central nervous system toxicity include metallic taste, perioral numbness, and tinnitus (Table 14.2).
Table 14.2
Potential complications of trigger point injections
Potential complications | |
|---|---|
Local anesthetics | Botulinum toxin |
• Allergic reaction | • Allergic reaction |
• Infection | • Infection |
• Hematoma | • Hematoma |
• Syncopal episode | • Syncopal episode |
• Intravascular injection | • Intravascular injection |
• Systemic toxicity | • Paralysis of nearby muscles |
– Metallic taste | • Bowel/bladder dysfunction |
– Perioral numbness | |
– Tinnitus | |
– Slurred speech and blurred vision | |
– Altered consciousness | |
– Convulsions | |
– Cardiac arrhythmias | |
– Cardiac arrest | |
Side effects of botulinum toxin injection include spread of paralysis beyond the injection site, potential bowel or bladder dysfunction, and flu-like symptoms. In the only randomized controlled trial of botulinum toxin injections for pelvic pain [13], there was no statistically significant difference in reported side effects between the botulinum toxin and placebo groups. However, two women in the botulinum toxin group did develop intermittent pelvic floor dysfunction that was self-limited [13]. Botulinum toxin should be used with caution in patients using other medications that affect neuromuscular transmission such as muscle relaxants and anticholinergic agents and in those with an underlying neuromuscular disorder [16].
Vaginismus and Pelvic Floor Muscle Spasm
Vaginismus is defined as recurrent or persistent involuntary contraction of the musculature surrounding the distal third of the vagina [17]. This condition usually interferes with sexual intercourse. In addition to pain, vaginismus can cause psychological distress and interpersonal difficulties, which is why it is classified as a sexual pain disorder [18]. Vaginismus can involve spasm of the perineal and/or levator ani muscles. Treatment of vaginismus and generalized levator spasm includes physical therapy, biofeedback, and use of muscle relaxants. There is also a growing body of evidence which suggests that botulinum toxin injections may offer another viable option for treatment (Table 14.3).
Table 14.3
Summary of the evidence for botulinum toxin use in gynecology
Authors | Study design | # of participants | Indication for use | Dose & type of botulinum toxin | Injection sites | Results |
|---|---|---|---|---|---|---|
Jarvis et al. [10] | Prospective cohort | 12 | Chronic pelvic pain, Pelvic floor spasm | 10 units botulinum toxin A injected at 4 sites; 40 units total | Puborectalis and pubococcygeous | Reduction in dyspareunia, dysmenorrhea, and pelvic floor pressures at 3 months |
Romito et al. [11] | Case series | 2 | Vestibulodynia, Pelvic floor spasm | 40–80 units, botulinum toxin A | Levator ani under EMG guidance | Symptom resolution at 3 months; benefits persisting up to 6 months |
Bertolasi et al. [12] | Prospective cohort | 39 | Vestibulodynia, Vaginismus | 20 units, botulinum toxin A | Levator ani under EMG guidance | Majority of patients needed repeat injections; symptom resolution in 63 % by 3 years after first injection |
Ghazizadeh and Nikzad [23] | Prospective cohort | 23 | Vaginismus | 150–400 units botulinum toxin A | Puborectalis, 3 injections on each side, total of 6 injections | Resolution of vaginismus on exam at 1 week post-injection in 96 % of patients. No recurrence at 12 months follow-up. |
Abbott et al. [13] | RCTa | 60 | Chronic pelvic pain, Pelvic floor muscle spasm | 20 units botulinum toxin A injected at 4 sites; 80 units total | Puborectalis and pubococcygeous | Reduction in dyspareunia pain scores from baseline for both botulinum toxin and placebo groups. Reduction in nonmenstrual pelvic pain for botulinum toxin group only. |
Dykstra and Presthus [19] | Prospective cohort | 12 | Vestibulodynia | 35 or 50 units of botulinum toxin A | Vestibule | Significant decrease in mean pain scores; duration of effect longer with higher dose |
Yoon et al. [20] | Prospective cohort | 7 | Refractory chronic pelvic pain, Vulvodynia | 20 units botulinum toxin A | Various sites in vestibule, levator ani, and perineal body | Significant improvement in pain scores. 5/7 patients required repeat injection. No recurrence at mean follow-up of ~1 year. |
Petersen et al. [21] | RCTa | 64 | Vestibulodynia | 20 units botulinum toxin A | Bulbospongiosis | Significant reduction in pain scores from baseline for both the botulinum toxin and placebo groups at 6 months post-injection. No significant difference between groups. |
The off-label use of botulinum toxin to treat levator spasm was first reported in 2004. Jarvis and colleagues [10] treated 12 women with a long-standing history of chronic pelvic pain and objective evidence of pelvic floor muscle hypertonicity with botulinum toxin type A (BOTOX, Allergan) injections. A total of 40 units were injected into 4 sites in the puborectalis and pubococcygeous under conscious sedation. At 3 months follow-up women experienced significant improvement in dyspareunia and dysmenorrhea. Pelvic floor resting pressures were also significantly reduced after injection. Although the maximum reduction in pressure was observed at 1 month post-injection, the mean resting pressure remained 25 % lower than baseline at 3 months post-injection.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


