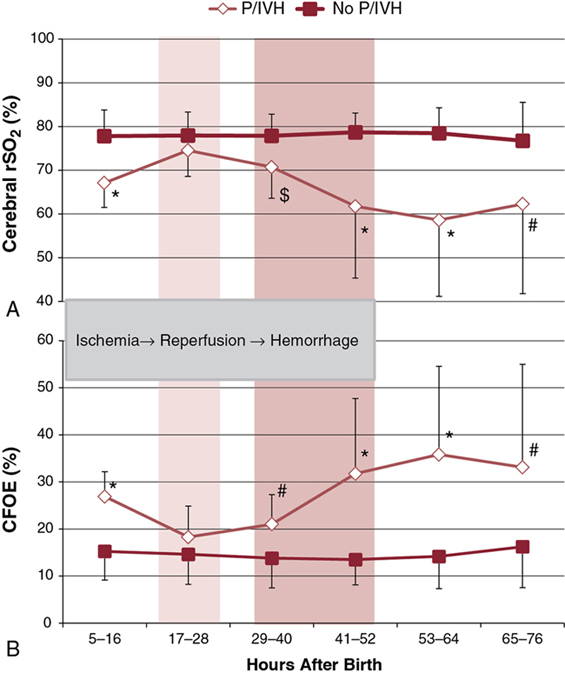
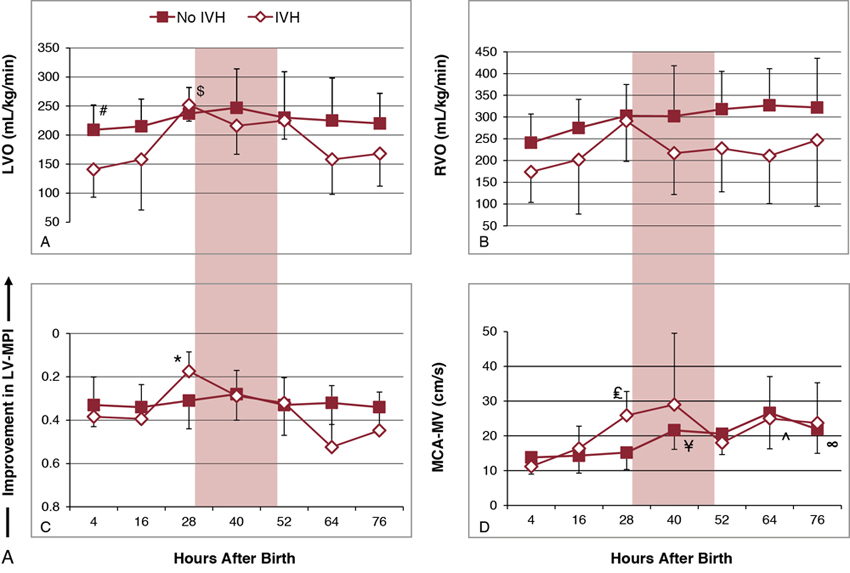
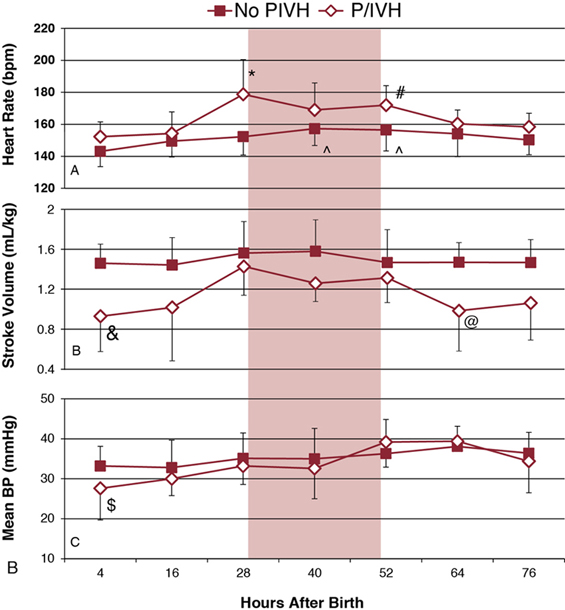
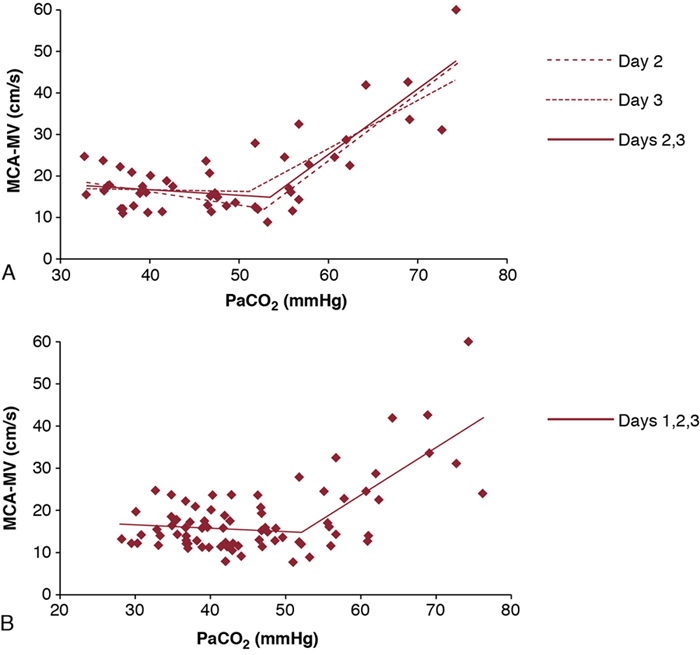
Shahab Noori, Istvan Seri Key points Peri-/intraventricular hemorrhage (P/IVH) is a devastating complication of prematurity that affects about a third of extremely preterm infants (<28 weeks’ gestation).1 P/IVH is a major risk factor for poor neurodevelopmental outcome, hydrocephalus, and mortality amongst these patients.2 Although the pathogenesis of P/IVH is complex and likely involves multiple different mechanisms, alteration in cerebral hemodynamics is thought to play a major role. Recent advances in noninvasive monitoring have highlighted the hemodynamic antecedents of P/IVH. This chapter reviews the inherent vulnerabilities of preterm infants during the transitional period and how the interaction between transitional hemodynamics and interventions aimed at supporting respiratory and cardiovascular function can increase the risk of P/IVH. As the physiology of fetal circulation is discussed in Chapter 1, we only provide a brief review of its main characteristics pertinent to the topic of this chapter. In utero, the most oxygenated blood, with oxygen saturation of around 75–85%, flows from the umbilical vein through the ductus venosus to the inferior vena cava (IVC). Due to mixing with venous blood from the portal and hepatic circulations in the liver and to some mixing with the venous blood flowing from the lower body in the IVC, the oxygen saturation of blood entering the heart is only about 70%. Of note, blood flowing in from the ductus venosus into the IVC is primarily diverted by the Eustachian valve toward the foramen ovale and into the left atrium.3–5 The low flow of poorly oxygenated pulmonary venous return to the left atrium admixing with this flow via the foramen ovale still ensures supply of relatively well-oxygenated blood to the heart and brain with an oxygen saturation around 60%. On the other hand, blood returning from superior vena cava (SVC) and the stream in the IVC, representing blood returning from the lower parts of the body, are preferentially directed to right ventricle. As most of right ventricular output is diverted through the patent ductus arteriosus (PDA) to the systemic circulation, both ventricles contribute to the systemic circulation. Given the low blood flow to the lungs, due to high pulmonary vascular resistance, left ventricular preload is relatively small. As such, during fetal life, the contribution of the right ventricle to systemic blood flow is greater than that of the left ventricle. In the fetus the combined cardiac output is about 400–450 mL/kg/min, with only about 11–25% constituting the pulmonary circulation.3,6–9 This is in contrast to postnatal circulation, where the left and right cardiac outputs are equal and average about 200 mL/kg/min. The low-resistance placental circulation facilitates the high cardiac output in the fetus by reducing the afterload. At birth, pulmonary vascular resistance drops precipitously as the newborn starts breathing and the lungs become the organ of gas exchange. This increases pulmonary blood flow and changes the ductal flow pattern in a way that progressively directs blood from the right ventricle to the pulmonary circulation.10 The increased pulmonary blood flow in turn increases left-sided preload and promotes functional closure of the foramen ovale. This, along with the closure of the ductus arteriosus over the following 2–3 days, transforms the circulation to the adult-type (postnatal) circulation, in which the pulmonary and systemic circuits are not functioning as parallel circulations anymore but as circulations in series. Despite its complexity, this transformation occurs smoothly in most term infants. However, in preterm infants, especially those born before 28 weeks’ gestation, this process is hindered by immaturity of the organ systems and is more likely to represent an abnormal cardiorespiratory transition. Accordingly, it is likely to be associated with circulatory compromise, as discussed in detail in this chapter. Understanding the evolution of cerebral hemodynamics from fetal circulation through the postnatal transition is critical in understanding the role of abnormal transition in pathogenesis of P/IVH. However, measurement of cerebral blood flow (CBF) is challenging in neonates, especially in preterm infants and during the immediate postnatal period. CBF has been assessed using radioactive xenon clearance, positron emission tomography, Doppler ultrasonography, magnetic resonance imaging, and near-infrared spectroscopy (NIRS). Each of these methods has its own intrinsic limitations. Due to their noninvasive nature and bedside availability, Doppler and NIRS are the most commonly used methods for the assessment of CBF. With Doppler ultrasonography, various surrogates of CBF, such as SVC blood flow and a major cerebral artery blood flow velocity, have been used to characterize intermittent changes in cerebral hemodynamics. On the other hand, NIRS allows for the continuous assessment of regional tissue oxygen saturation (rSO2) or tissue oxygenation index (TOI). Although NIRS does not measure blood flow directly, by considering cerebral regional oxygen saturation (CrSO2), clinical information, and certain other parameters, changes in CBF can be deduced. Considering arterial oxygen saturation (SPO2), the index of cerebral fractional oxygen extraction (CFOE) can be calculated according to the following formula: (SPO2 – CrSO2)/SPO2. CrSO2 has a direct and CFOE an inverse relationship with changes in CBF. In other words, a reduction in CrSO2 or an increase in CFOE indicates a decrease in CBF provided certain assumptions hold true. These assumptions include no significant changes in SPO2 (with CrSO2), organ metabolism, hemoglobin, and/or the distribution of blood in tissue among arteries, veins, and capillaries. During fetal development, brain blood flow increases both as an absolute value and per gram of tissue.11,12 Animal and human studies have shown a decrease in CBF at and immediately after birth.13,14 The cause of this reduction is unclear but in part may be related to an increase in tissue oxygenation at birth compared to the fetal life.10,13 Interestingly, the progressive change of PDA flow pattern from right to left to left to right during the first few minutes after birth strongly and inversely correlates with middle cerebral artery mean blood flow velocity (MCA-MV), a surrogate of CBF.10 This suggests a possible role of PDA immediately after birth in reduction of CBF. Alternatively, the changes in PDA flow pattern and reduction in CBF may be independent, and both reflective of the increasing oxygen tension following delivery. More data are needed to elucidate the normal changes in cardiovascular function and cerebral hemodynamics at and immediately after birth, especially in preterm infants. After the immediate postnatal period, CBF increases rather significantly over the following days and more gradually afterward in both preterm and term infants.15–18 Despite the rise in CBF, it remains at only a fraction of the adult value.19,20 Moreover, sick preterm infants have even lower CBF.19 The low CBF in neonates may be explained by lower brain metabolism; however, cardiovascular maladaptation in preterm infants may also contribute to the observed low CBF. Preterm infants who develop P/IVH have lower CBF after birth. Studies using NIRS found higher CFOE on the first postnatal day in preterm infants who later develop P/IVH.21 Serial measurements of SVC flow, a surrogate for CBF, also found that low SVC blood flow in the first few hours after birth is a risk factor for P/IVH.22 However, caution needs to be exercised when using SVC flow as a surrogate of CBF since, in the preterm neonate, it is estimated that only around 30% of the blood flow in the SVC represents blood coming back from the brain (Chapter 2). The last two decades have seen an increased use of ultrasonography by neonatologists to elucidate the cardiovascular adaptation during the transitional period. In addition, advances in monitoring technology have brought NIRS to a more widespread use in research and also have facilitated its introduction into clinical care. The newer NIRS sensors have allowed for more continuous and prolonged monitoring of regional tissue oxygen saturation and for quick applications of the sensors in situations when time is of the essence, for example, in the delivery room. Therefore, despite the limitations mentioned earlier, increased application of the newer NIRS technology and ultrasonography have provided valuable insights into the cerebral hemodynamic changes that precede P/IVH. A recent nested case-control study compared CrSO2 and CFOE between preterm infants with and without P/IVH during the first 15 days after birth.23 Measurements were done daily for 2 hours for 8 days and then on day 15.23 The authors found lower CrSO2 and higher CFOE in the P/IVH group, suggestive of lower CBF throughout the first 8 days. While low CBF in the first postnatal day has consistently been reported, its persistence for a week has not.17,21,22,24 In contrast, another nested case-control study monitoring cerebral oxygenation during the first few postnatal days found higher CrSO2 and lower CFOE during the 24 hours prior to detection of severe P/IVH.25 In other words, this study suggests that higher rather than lower CBF precedes brain hemorrhage. This discrepancy may be explained by the differences in the timing and duration of monitoring between the two studies.26 In a recent study, in addition to performing frequent and regularly timed head ultrasounds and echocardiography, we prospectively and continuously monitored NIRS indices in extremely preterm infants (<28 weeks) during the first 3 postnatal days. The study found a unique pattern with identifiable phases of changes, among others, in the indices of CBF in patients who developed P/IVH (Figure 7.1).27 The CrSO2 and CFOE were indicative of low CBF in the earlier hours of the first postnatal day, followed by a period of an increase in CBF before detection of P/IVH around 48 hours after birth and, thereafter, a subsequent decrease in CBF. These findings suggest that there are two distinct hemodynamic phases in the pathogenesis of P/IVH: an early hypoperfusion and a later reperfusion phase. The prospective, comprehensive, and continuous design of the study allowed for detection of the two phases. Given the dynamic changes in CBF over the first few days, intermittent or a short period of monitoring will miss either the hypoperfusion or reperfusion phase.26 In the remainder of this chapter we will discuss the vulnerabilities of preterm infants during the transitional period that increase the risk of P/IVH and focus on the causes and risk factors that lead to or potentiate the hypoperfusion and/or reperfusion phases. The brain of a preterm infant is vulnerable to development of P/IVH due to both structural and functional immaturity. The germinal matrix is the site of active proliferation of future neuronal and glial cells and, as such, is a highly vascularized and metabolically active tissue.28 Its capillary network consisting of thin-walled fragile vessels is susceptible to rupture.29 The germinal matrix involutes between 28 and 34 weeks.28,30 Therefore, until its final involution, the germinal matrix is susceptible to bleeding, especially in preterm infants <28 weeks’ gestation. In addition, the germinal matrix lies within an arterial end zone, which makes it particularly vulnerable to hypoperfusion-reperfusion injury.31 The immature venous system is prone to congestion, which further increases the risk of hemorrhage in this population.28,30 The ability to maintain CBF relatively constant despite fluctuations in blood pressure (i.e., CBF autoregulation) is an important protective mechanism against ischemia and hyperperfusion (Chapter 2). The correlation between mean blood pressure and tissue oxygenation index or regional tissue oxygen saturation using NIRS allows for continuous assessment of CBF autoregulation by analyzing coherence and transfer function gain. Coherence is a measure of linear correlation between blood pressure and an index of CBF (i.e., cerebral oxygenation), and therefore an arbitrary cutoff, usually 0.5, is used to define presence or absence of autoregulation. On the other hand, transfer function gain assesses the degree of impairment by measuring the effects of changes in the amplitude of the blood pressure waveform on the amplitude of changes in cerebral tissue oxygenation. Both methods have their strengths and limitations and are not routinely used in clinical settings (Chapter 2).32 The brain of preterm infants has immature CBF autoregulation.33–35 In addition, most very preterm neonates have mean blood pressures very close to the lower elbow of the blood pressure autoregulatory curve during the first 24 hours after delivery (Chapter 2). Although CBF autoregulation is present even in the most immature preterm infant, the autoregulatory plateau is quite narrow. Moreover, immaturity per se and/or the interventions aiming to support these patients can result in systemic changes that alter CBF autoregulation (see hypotension and permissive hypercapnia). Given the role of CBF autoregulation in ensuring maintenance of adequate CBF and prevention of hyperperfusion, immature and impaired autoregulation has long been considered a risk factor for P/IVH. Indeed most, but not all, studies of CBF autoregulation in preterm infants have shown an association between impaired autoregulation and the occurrence P/IVH.33–38 Another vulnerability of the brain of the very preterm neonate (≤28 weeks’ gestation) is the immaturity of the forebrain (including cortex, thalamus, hypothalamus, basal ganglia) vasculature displaying characteristics of the blood flow regulation of a non-vital organ during the first postnatal days. In other words, the vessels of the forebrain respond with vasoconstriction to decreasing perfusion pressure or hypoxia rather than with vasodilation as expected for the vessels of a vital organ (brain, heart, and adrenal gland).39 Several lines of evidence support this notion.40–42 For example, beagle pups exposed to hypoxia exhibit vasodilation of the hindbrain (medulla, pons, cerebellum) but vasoconstriction of the forebrain.40 In humans CBF autoregulation appears in the brainstem first and in the forebrain only later in gestation.41 Therefore vital organ assignment appears to be developmentally regulated, with the forebrain lagging behind the hindbrain in acquiring the properties of a high-priority vascular bed. The myocardium of a neonate, even at term, is immature and quite different than that of older children and adults. It has more water content and less contractile elements. In addition, the immature sarcoplasmic reticulum makes cytosolic calcium the primary source of second messenger, calcium for myocardial function. These differences affect both systolic and diastolic functions. Indeed, the neonatal myocardium is more sensitive to afterload; specifically, its lower compliance adversely affects ventricular filling and is dependent on extracellular calcium for its function. Preterm infants, especially during the transition, exhibit a cardiovascular response to acidosis that is different from that of adults. A recent study in hemodynamically stable very preterm infants during the transitional period showed that, while acidic pH was not associated with a decrease in myocardial contractility, cardiac output failed to increase, presumably because the expected acidosis-associated decrease in systemic vascular resistance didn’t take place.43 It has been postulated that immaturity of the myocardium predisposes preterm infants to a low systemic flow state22,44 and therefore contributes to the low CBF observed in a subset of very preterm neonates who later develop P/IVH. If we consider SVC flow as a surrogate for systemic flow, the finding of low SVC flow in the P/IVH group represents low cardiac output in the immediate postnatal period, with the fetal channels open.22 Indeed, low left (LVO) and right ventricular output (RVO) have been shown to be prevalent in those who later develop P/IVH (Figure 7.2).27 However, the underlying causes of this low cardiac output are unclear. Among others, a suddenly increased high afterload following removal of low-resistance placental circulation in the setting of an immature myocardium has been postulated to be one of the underlying causes of this finding. Indeed, there is a difference in the inverse linear relationship of contractility and afterload among preterm infants with normal and low SVC flow, with patients having low SVC flow as a group exhibiting a steeper regression line suggestive of lower contractility in this group.44 However, this is not a consistent finding and a recent study found no difference in afterload or load-dependent and load-independent indices of contractility between patients who develop P/IVH and those who don’t.27 Whether low preload could explain the observed low cardiac output is not known, in part because of the difficulty in assessing preload using noninvasive techniques. However, recent studies of delayed cord clamping and cord milking suggest a possible role for low preload in the observed low cardiac output state (see below). Thus it is likely that the low cardiac output is multifactorial, with abnormalities in preload, contractility, and afterload and other factors such as ductal shunting contributing to a varying degree in different patients. Following the initial low flow state, cardiovascular function improves, and cardiac output normalizes. This improvement in systemic flow is also associated with reperfusion of the brain and precedes occurrence of P/IVH (Figure 7.2).27 Recent studies of timing of cord clamping show enhanced hemodynamic status and possible lower rate of P/IVH when clamping of the umbilical cord is delayed for 30–60 seconds, although in more recent studies there is less association seen with cord clamping and P/IVH.45–48 These data suggest a better postnatal transition with delayed cord clamping. A more gradual separation from the low-resistance placental circulation might facilitate the adaptation of the left ventricle to the postnatal increase in afterload. Prolonging the time of cord clamping also promotes placental transfusion. In the term neonate it is estimated that 16–23 mL/kg of blood is transfused from the placenta to the neonate, with a 1- and 3-minute delay in the timing of cord clamping, respectively.49 Importantly, the onset of breathing before cord clamping promotes earlier establishment of pulmonary blood flow, and thus better maintains left ventricular preload, and increases placental transfusion to the newborn.50 Although all of the above mechanisms are likely contributory, increased intravascular volume due to placental transfusion has been speculated as the most important cause for the better transition in preterm infants with delayed or physiologic cord clamping. This speculation is based on the finding that similar cardiovascular benefits are seen with milking of the cord.51–53 Preterm infants receiving placental transfusion by milking of the cord have higher cerebral oxygenation and SVC flow, cardiac output and urine output, and a decreased incidence of hypotension and are less likely to receive vasopressor-inotropes or inotropes compared to those with immediate cord clamping.51–53 However, the finding of a higher rate of P/IVH among the extremely preterm infants randomized to cord milking in a recent RCT has raised significant concerns about the safety of this procedure intended to be used to facilitate placental transfusion.54 The underlying mechanisms for the increase in P/IVH are unknown. Although there was no indirect evidence of an increase in CBF with cord milking in patients monitored with NIRS in the delivery room,55 it is possible that in vulnerable individuals, a rapid increase in the preload, and thus CBF, may play a role. Historically, hypovolemia was thought not to be prevalent in the newborn, given the absent or weak relationship between measured blood volume and blood pressure. However, the finding of a more stable cardiovascular status in preterm infants with placental transfusion, either from delayed cord clamping or cord milking, indicates that hypovolemia may indeed play a role in hemodynamic instability during the immediate transition period and, as such, might contribute to pathogenesis of P/IVH. Indeed, the recent report of an association between lower initial hematocrit used as a surrogate for intravascular volume and the occurrence of P/IVH supports the role of hypovolemia in the pathogenesis of P/IVH.56 As mentioned earlier, preterm infants who develop P/IVH have lower CBF in the first hours after birth, as suggested by lower CrSO2 and SVC flow. The findings of a higher CrSO2 and SVC flow in patients with delayed cord clamping or cord milking suggest that the incidence of low CBF occurring immediately after delivery can be reduced. Thus enhancing placental transfusion may, at least in theory, be associated with a lower incidence of P/IVH. While reduction in cerebral hypoperfusion with placental transfusion appears to decrease the predisposition to P/IVH, cord milking likely increases the risk of P/IVH.54 Although the mechanism(s) leading to the increased occurrence of P/IVH with cord milking are unclear, the use of this intervention is currently not recommended.57 Furthermore, the importance of the onset of breathing in delayed/physiological cord clamping also suggests that a gradual, smoother shift from placental blood flow to pulmonary blood as the source of left ventricular preload immediately after birth is among the key contributors associated with an improved hemodynamic status in the very preterm neonates during early transition. Although a large PDA with and without hypotension during early transition has been associated with P/IVH,22,58,59 the extent of the contribution, if any, of the PDA to the pathogenesis of P/IVH is unclear. As discussed in Chapter 2, the pattern of CBF is affected by a PDA. Moreover, with an inadequately compensated cardiac output for the degree of the left-to-right PDA shunt, CBF is also reduced. Most studies assessing cerebral oxygenation using NIRS have shown a lower CrSO2, with a PDA and improvement after closure of the ductus arteriosus.60–62 The increased stroke volume and, as a result, the left ventricular output represent a compensatory mechanism to attenuate the effect of the shunt on systemic perfusion, especially pre-ductally. This adaptive process may be limited in a subset of patients, especially during first few hours after birth. As discussed earlier, this is the period when cerebral ischemia is prevalent in patients who later develop P/IVH. Indeed, there is a temporal relationship between a hemodynamically significant PDA and low indices of CBF during this critical period.10,22 Authors of earlier studies showing a reduction of the incidence of P/IVH with indomethacin prophylaxis suggested that early PDA might have a role in development of P/IVH. However, lack of any effect of prophylactic ibuprofen on the incidence of P/IVH, despite a significant reduction in PDA rate, leads to the casting of doubt on the role of early PDA in the development of P/IVH and suggests a more direct effect of indomethacin on cerebral hemodynamics. Nevertheless, the strong relationship between a significant PDA and low CBF and CrSO2 does suggest that PDA may be a contributing factor to the ischemic phase preceding the development of P/IVH. Hypotension is common in preterm infants during transition. Hypotension is discussed in detail in Chapter 3; suffice to say that there are many reasons for a preterm infant to be hypotensive during the transitional period. These include postnatal maladaptation superimposed on immaturity of the cardiovascular system, inadequate compensation for the PDA shunt, prevalence of relative adrenal insufficiency, increased rate of sepsis, acidosis, and the detrimental impact of inappropriate ventilatory support on neonatal hemodynamics. Hypotension has long been recognized as a risk factor for P/IVH.63–66 Given the fact that hypotension is a hallmark of uncompensated shock, the association between hypotension and P/IVH is not surprising. However, the uncertainty about the definition of hypotension and the influence of other coexisting variables (see Chapter 3) make it difficult to propose a “safe” blood pressure range. Over the past decade, there has been an increasing concern about the possible role of treatment of hypotension in the occurrence of P/IVH and poor neurodevelopmental outcome associated with hypotension.67–69 This is indeed a possibility, at least theoretically. By definition, hypotensive preterm infants are outside the CBF autoregulatory range, where cerebral perfusion is pressure passive.70,71 Therefore inappropriate titration of vasopressor-inotropes can increase blood pressure and brain blood flow70,72 and thus, in theory, increase the risk of P/IVH (Chapter 3). However, of note is that, although careful, stepwise titration of vasopressor-inotropes for hypotension restores brain blood flow along with the blood pressure, CBF autoregulation does not regain its functionality for a period of time.70,72 Yet, there are data, although not conclusive, on the potential beneficial effects of careful titration of cardiovascular medications.64,73,74 A retrospective study of dopamine-treated preterm infants <28 weeks’ gestation found that failure to respond to dopamine was associated with almost a sixfold greater likelihood of developing P/IVH. On the other hand, a strong response to dopamine was associated with a reduction in risk of P/IVH.74 In contrast, the secondary analysis of a recent RCT of peripheral perfusion–based approach versus blood pressure–based approach to circulatory management of the preterm infants during the transitional period found a higher incidence of P/IVH in the subset of the blood pressure–based management group who had responded to the treatment (volume, pressors, and/or inotropes).75 Although the reasons for these findings are unclear, differences in the choice of medication and the approach to drug titration to avoid significant and rapid fluctuations in blood pressure may have played a role.70,76 During the last decade, the trend toward less aggressive treatment of hypotension77 has provided a glimpse into the potential effects of sustained hypotension in patients without clinical evidence of poor perfusion (“isolated hypotension”). For example, the analysis of the French national prospective population-based cohort study allowed for the matching of 119 extremely preterm infants with untreated, “isolated hypotension” to 119 neonates who received treatment despite also having no clinical evidence of poor perfusion.78 Accordingly, none of the patients included in this study had any clinical sign of inadequate cardiovascular function other than hypotension. Hypotension was defined as a mean blood pressure less than gestational age in weeks during the first 3 postnatal days. The findings revealed that the group treated for the “isolated hypotension” had a higher rate of survival without severe morbidity and a lower rate of severe P/IVH and cerebral injury. Interestingly, the association between treatment and better outcome was even stronger when hypotension was defined as a mean blood pressure in mmHg less than gestational age in weeks by more than 5 points. Although this dose-effect relationship strengthens the possibility of causality, further studies are clearly needed to verify the impact of hypotension on P/IVH and long-term outcome. It is likely that the underlying pathophysiology of brain injury associated with hypotension is multifactorial and that low CBF due to low perfusion pressure, inappropriate treatment, and titration of vasopressor-inotropes resulting in intermittent cerebral hypo- and hyperperfusion and other coexisting factors independent of hypotension all contribute to varying degrees to the development of P/IVH. The fact that hypotension is almost always treated,79 albeit at different thresholds, makes establishing causality for adverse outcomes and defining the extent of contribution of various aspects of clinical care to the development of P/IVH in response to hypotension nearly impossible. There are many challenges in designing a study to ascertain the impact of each of these factors in the pathogenesis of P/IVH and poor neurodevelopmental outcome. These include the inability to utilize a physiology-based and individualized definition of hypotension for each patient, the heterogeneous etiology and pathophysiology of hypotension (i.e., abnormality in preload, contractility, afterload and/or vascular tone dysregulation), and complexity of selecting the appropriate treatment for the given pathophysiology and clinical presentation. In addition, given the strong belief among neonatologists of harmful effect of hypotension and difficulties in recruitment, conducting randomized control trials with a no-treatment arm does not appear feasible.79,80 The close anatomic and physiologic relationship between the respiratory and cardiovascular systems results in the notion that changes in the two systems mutually affect one another. Due to the vulnerability of the preterm infant, these interactions are particularly important during the early postnatal transitional period. Most very preterm infants require some level of respiratory support for immaturity of the lungs and the respiratory center. The impact of positive airway pressure and invasive or noninvasive ventilation have been studied in animals and to a lesser extent in humans. Animal studies demonstrate that with increasing positive end-expiratory pressure, pulmonary vascular resistance increases, thereby raising right ventricular afterload, resulting in reductions in RVO.81,82 In addition, the increased intrathoracic pressure-driven decrease in systemic venous return further reduces RVO and, as a result, systemic blood flow also decreases. Studies in human neonates have reported inconsistent results, with some findings being similar to those obtained in animal models, albeit with a milder impact, while others show no effect.83–86 The reason for this discrepancy is unclear but likely involves limitations of assessment tools and differences in lung compliance among the patients in the different studies. The latter reason could explain the significant hemodynamic alteration observed in animal models where lung compliance is normal and therefore intrathoracic pressure is more readily transmitted to the vasculature and the heart. Although the effect of positive airway pressure in the range commonly used in clinical practice appears to be relatively small, inappropriately high pressure for the degree of lung disease can reduce systemic blood flow and also lead to increased cerebral venous pressure, both of which may contribute to pathogenesis of P/IVH. Indeed, a recent study showed that an altered internal cerebral venous flow pattern is associated with an increased rate of P/IVH.87 This increase in P/IVH is presumably due to the effect of elevated intrathoracic and right atrial pressures on cerebral venous pressure. However, other variables, including high blood pressure and a PDA, have also been associated with altered internal cerebral venous flow.88 Given the prevalence of less effective or impaired CBF autoregulation in sick, extremely preterm infants, decreases in systemic perfusion can relatively easily lead to a drop in CBF. In addition, the fluctuation of CBF associated with mechanical ventilation, perhaps due to asynchrony, increases the risk of P/IVH.89 While positive pressure ventilation increases central and cerebral venous pressure, tension pneumothorax can result in a significant, abrupt rise in these pressures and increases the risk of P/IVH.90,91 Surfactant administration can also impact CBF initially by altering carbon dioxide levels and subsequently by the hemodynamic consequences of potential lung hyperinflation following improvement in lung compliance, provided that ventilator support has not been weaned in a timely fashion.92–94 Tracheal suction can also alter cerebral hemodynamics by increasing both arterial and venous blood pressure. In addition to the direct effects of ventilatory support, treatment strategies such as permissive hypercapnia with the resultant acidosis can impact cardiovascular function and CBF (see below). Carbon dioxide has a potent effect on the vascular system in general and brain in particular. Increases and decreases in partial pressure of CO2 (PaCO2) cause cerebral vasodilation and vasoconstriction, respectively. In fact, changes in PaCO2 are more potent regulators of CBF than are changes in blood pressure, even outside the autoregulatory blood pressure range (Chapter 2). Therefore hypocapnia and hypercapnia may impact the hypoperfusion and reperfusion phases preceding P/IVH, respectively. Although hypocapnia is associated with ischemic brain injury and periventricular leukomalacia,95,96 it does not appear to play a significant role in pathogenesis of P/IVH.97 This could be due to absent or low reactivity of CBF to changes in PaCO2 during the first postnatal day, a period when hypoperfusion is commonly reported in this population. Indeed, there is an evolution of the CBF-CO2 reactivity in preterm infants with higher responsiveness of the cerebral vasculature to changes in PaCO2 with each passing day during the first few postnatal days.98,99 In one study the CBF reactivity per kPa (7.5 mmHg) change in PaCO2 was about 11% on the first postnatal day compared to 32% on the second day.100 A weak relationship between PaCO2 and CBF on the first postnatal day has also been shown using NIRS.101 We found no relationship between MCA-MV, a surrogate for CBF, and PaCO2 on the first postnatal day and a progressively stronger positive linear relationship on postnatal days 2 and 3 in preterm infants.99 On the other hand, hypercapnia has consistently been shown to be associated with P/IVH.97,102–104 The highest PaCO2 during the first 3 postnatal days has a dose-dependent relationship with odds of developing P/IVH.102 Although cause-and-effect relationship has not been established, the increase in CBF with hypercapnia can accentuate the reperfusion phase preceding the occurrence of P/IVH.27 In addition, hypercapnia attenuates CBF autoregulation. A study of the correlation between blood pressure and MCA-MV demonstrated a steeper coefficient line with higher PaCO2 values suggesting a progressive impairment of CBF autoregulation with rising PaCO2 above normal.105 Similarly, we found no relationship between MCA-MV and blood pressure in normotensive preterm infants but, when adjusted for PaCO2 levels, a significant positive relationship emerged, suggesting that higher PaCO2 is associated with impairment of CBF autoregulation.99 Furthermore, we found a breakpoint in the relationship between PaCO2 and MCA-MV with no relationship below PaCO2 values of 52–53 mmHg and a strong positive linear relationship above this cutoff (Figure 7.3).
Chapter 7: Transitional hemodynamics and pathophysiology of peri-/intraventricular hemorrhage
Introduction
Fetal and transitional circulation
Cerebral blood flow
Normal changes in CBF
CBF and P/IVH
Vulnerabilities of preterm infants during transition
Inherent vulnerability of the immature brain
Immature myocardium
Hypovolemia and timing of cord clamping
Patent ductus arteriosus
Hypotension
Cardiorespiratory interaction
Hypocapnia and hypercapnia
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Obgyn Key
Fastest Obstetric, Gynecology and Pediatric Insight Engine
