Vulvovaginal lichen planus. Note the subtle erosions and heavy vaginal discharge.
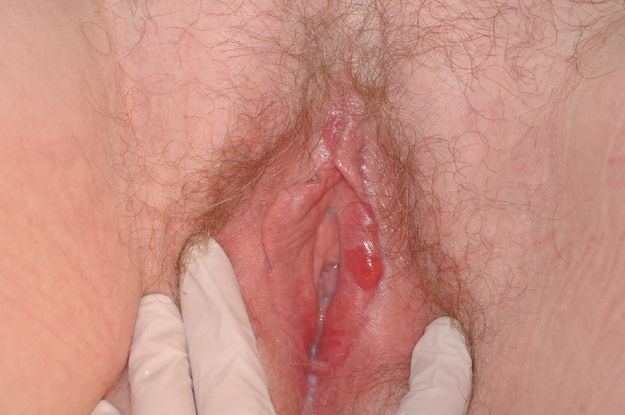
Vulval lichen planus. Note the more typical glazed erosions.
Lichen planus can occur on any part of the skin. In addition to causing vulval disease, it may also cause itchy skin rashes, nail dystrophy, hair loss and oral and other mucosal lesions. The clinical presentation is highly variable.
The type of lichen planus that involves the vulva and vagina (also known as mucosal lichen planus) is the commonest type of lichen planus that causes vulval disease (Figure 5.1). When lichen planus involves the genital area, it often also involves the oral mucosa, although not invariably. Rarely, it may extend into the oesophagus and anal canal.
The common type of cutaneous lichen planus presenting with itchy violaceous papules with white streaks called Wickham’s striae occurs on the vulva (Figure 5.2) but is very uncommon compared with the mucosal type of lichen planus.
It is almost always a disease of adults and is very rare in children and adolescents. The majority of women with vulval lichen planus are middle-aged and beyond.
Presentation
The typical patient with vulval lichen planus is in her 50s and beyond; however, it can occur in women in the second or third decade of life onwards, and in young patients it is a devastating diagnosis. It is exceptionally rare in children.
Patients with vulval lichen planus usually present with pain, dyspareunia and heavy but non-offensive discharge. Itch is not a prominent feature. Dyspareunia is usually severe, and patients have often become completely apareunic. If the anal canal is involved, pain with defecation is characteristic.
When oral disease is also present, patients notice oral soreness and sensitivity as well as tenderness, raw areas and gingivitis.
Aetiology
The pathogenesis of lichen planus is T-cell related, but the exact nature of the disease remains unknown. In a few cases, it is related to hepatitis B and C virus infection, and has been reported to be precipitated by hepatitis B virus vaccination.
Older texts report an anecdotal association with photographic film-developing agents, and oral lichen planus has been associated with amalgam fillings. However, most cases are idiopathic.
Examination
Physical examination is often confusingly non-specific, ranging from non-specific erythema to frank ulceration. The hallmark of lichen planus is erosion of the mucosal surface of the introitus, extending into the vagina and involving the cervix. This presents as glazed erythema, and if one looks closely, the loss of epithelium is evident. Interspersed with this bright erythema you may notice grey patches. This is a classic clinical finding that is very helpful but unfortunately not invariable.
If lichen planus is not diagnosed and treated early, scarring will usually occur. On the vulva, this may involve loss of the labia minora, labial fusion (either anteriorly or posteriorly) and complete obliteration of the clitoris by clitoral hood adhesions. Patients with lichen planus may suffer from recurrent abscess formation because of this scarring, especially on the clitoris. Lichen planus can involve the vagina, and scarring here may result in occlusion or stenosis, making speculum examination impossible.
The distribution of the eruption is usually confined to the labia minora, the sulcus between the majora and minora, the introitus, the perineum and the vagina. However, extension to the perianal area and the anus may occur. As a result, the anal mucosa may also appear brightly erythematous and may also scar and stenose.
Lichen planus is highly treatment resistant, and patients do not respond to weak topical corticosteroids.
Investigation
Lichen planus has a classic histological appearance and is usually reported as being characterised by a predominantly lymphocytic infiltrate obscuring the dermoepidermal junction. This classic histopathology is easily found in non-genital skin and oral biopsies. Unfortunately, it may be difficult to obtain a diagnostic biopsy from the vulva.
When performing a vulval biopsy to make the diagnosis of lichen planus, never take the sample from an eroded area. If there are grey patches, these are often the most diagnostic.
If your clinical impression is that your patient has lichen planus and your biopsy does not confirm it, it may be necessary to make a clinical diagnosis.
Management
The initial treatment of lichen planus in all cases is with topical and/or oral corticosteroids. Lichen planus is a corticosteroid-responsive condition, but vulval lichen planus must be treated with a potent, not weak, corticosteroid.
The milder forms of vulval lichen planus are able to be controlled by topical steroid use alone, although this needs to be a potent preparation applied daily. We have observed a subset of patients with this disease, usually elderly women, whose condition is confined to the vestibule and who do very well with moderate cortisone ointments applied daily. Even over long periods of time running into years, this is effective and without side effects.
However, the more severe cases of lichen planus often require much more aggressive treatment, including oral prednisone. Because lichen planus requires strong topical and sometimes oral corticosteroids, it is important to introduce a steroid-sparing agent as soon as possible because of the potential for side effects.
Steroid-sparing agents that are available include:
Topical tacrolimus
Oral retinoids
Oral weekly methotrexate
Oral azathioprine
Oral mycophenolate
The details of how to use these agents are beyond the scope of this text. If you suspect that your patient has lichen planus, referral to a specialist is highly recommended. We do not recommend treatment of vulvovaginal lichen planus in general practice.
Surgical Treatment
Scarring and stenosis often require correction, but this must only be attempted once medical treatment has stabilised the disease.
Follow-Up
Once lichen planus is diagnosed, it is usually a long-term condition that must be controlled. Our aim is to induce remission with oral and/or topical therapy and to tailor maintenance therapy to suit the individual, minimising this as much as possible.
In our experience, the majority of patients can achieve good control, but it may take up to a year or even longer to achieve this. Not all patients are able to achieve painless intercourse, and a high degree of motivation is required.
Lichen planus is often a devastating and life-changing event for patients, necessitating a huge adjustment in lifestyle including daily medication, some of which involves significant risk and loss of sexual enjoyment and activity. The impact on the patient should never be underestimated.
Differential Diagnosis
Both lichen planus and lichen sclerosus are scarring conditions where loss of vulval architecture is typical. There are, however, important differences (see Table 5.1).
| Lichen planus | Lichen sclerosus | |
|---|---|---|
| Anatomical involvement | Vulva and/or vagina | Vulva only; never involves vagina |
| Erosive condition | Yes, primarily erosion of the mucosal surface of the introitus | Not primarily; however, may split or ulcerate as a complication of the disease |
| Appearance | Red and erythematous with patches of grey | White; redness only seen with superimposed psoriasis, candidiasis or corticosteroid-induced dermatitis |
| Childhood | Extremely rare | Occurs in children |
| Association with vulval cancer | Anecdotally associated with cancer, but association controversial | Association well documented |
| Treatment | Highly treatment resistant | Responds readily to treatment |
Other differential diagnoses include:
Graft-versus-host disease
Any autoimmune bullous disease, but particularly mucosal pemphigoid and bullous pemphigus
Desquamative inflammatory vulvovaginitis
Fixed drug eruption of the vulva
These, and others, are described below.
Graft-versus-Host Disease
This is the condition that most closely mimics vulval lichen planus. Patients with known chronic graft-versus-host disease not uncommonly experience mucosal involvement indistinguishable from lichen planus. Treatment is very similar.
Graft-versus-host disease occurs in 20–30% of stem-cell transplant recipients and is largely a skin disease. Chronic graft-versus-host disease often occurs in the mouth but tends to be overlooked in the vagina, where it may cause significant scarring and make intercourse impossible. It is often asymptomatic until intercourse is attempted for the first time (often many months post-transplantation).
Even when vaginal graft-versus-host disease is symptomatic, patients are often told that they have ‘thrush’ or a similar minor complaint. It is therefore essential that all female stem-cell transplant recipients are examined at 3–6 months after their transplant, even if asymptomatic.
Only appropriately experienced specialists, in close conjunction with the treating haematologist, should manage these patients.
Desquamative Inflammatory Vulvovaginitis (non-infective chronic vulvovaginitis)
Desquamative inflammatory vulvovaginitis (DIV) (Figure 5.3), also known as non-infective chronic vulvovaginitis, is a relatively recently described and uncommon condition. Its aetiology remains unknown and controversial. Very little has been written about it. It is not actually an erosive condition, but because it is painful and is very often confused with lichen planus, which may be erosive, it will be discussed here.

Desquamative inflammatory vaginitis with a typical petechial rash in the vaginal introitus.
We believe that DIV is probably the same entity as Zoon’s vulvitis and plasma cell vulvitis. All of these names create confusion, and it would probably be best for international consensus to provide an overarching term.
In practice, although very uncommon, it is still more common than lichen planus.
Desquamative inflammatory vulvovaginitis is essentially a non-infective, non-erosive chronic vulvovaginitis. Like lichen planus, with which it is often confused, it involves the vagina and mucosal surface of the labia minora. However, unlike lichen planus, it does not extend any further, nor does it involve the skin or the oral mucosa, and it does not produce scarring.
Desquamative inflammatory vulvovaginitis was first described 50 years ago. More recently, it was characterised by Professor Jack Sobel, a recognised expert on vaginitis, as a non-infective vulvovaginitis with a typical clinical appearance of erythematous glazed and/or petechial patches on the mucosal surface of the labia minora (Figure 5.3).
Sobel originally described a loss of normal vaginal homeostasis with loss of lactobacilli and predominance of cocci on vaginal cytology. However, the aetiology of this condition has yet to be determined, and studies involving more advanced techniques that might seek to characterise the microbiome of the vagina in patients with this condition have not yet been published.
Sobel established diagnostic criteria based on a case series of 51 patients. These were:
2. Purulent exudate.
3. Increased parabasal cells on wet film.
5. Gram stain showing relative loss of Gram-positive bacilli and the presence of Gram-positive cocci together with the presence of polymorphonuclear leukocytes.
Desquamative inflammatory vulvovaginitis is not usually a disease of young women and has never been described in a child. The typical patient is in the fourth decade and beyond.
Presentation
The symptoms are usually a combination of soreness, dyspareunia and discharge (occasionally bloody). Itch is not a prominent feature and scarring is never encountered.
The appearance of the vulvovaginitis varies from non-specific confluent dull or glazed erythema, to patchy erythema, to petechiae and petechial patches. A non-offensive, greenish discharge is often but not always present. The rash is never found beyond the labia minora. Although on first inspection the rash may give the impression of being erosive, there is no true breach of the epithelium.
Investigation
Microscopy may reveal polymorphs and loss of lactobacilli, both of which are not specific to this condition. Vaginal cultures do not show any recognised pathogen associated with vaginitis. Group B Streptococcus is a frequent isolate, but in this context is not pathogenic. All these findings are by no means invariable.
Published studies such as the one by Sobel cite changes on examination of vaginal wet mounts. The problem for the average practitioner is that most of us possess neither a microscope in our office nor the skills (or time) to interpret wet mounts.
There have been few studies describing the histological features of DIV. Those that exist detail either a non-specific inflammatory response or an interface dermatitis with a heavy mixed inflammatory infiltrate, which obscures the dermoepidermal junction. The pattern is different to that seen in lichen planus. In general, we do not biopsy these patients because of the non-specific nature of the histopathology.
In 2010, we published a case series of 101 patients with DIV. We found that 56% of them had historical triggers, most frequently chronic diarrhoea or antibiotic treatment, while 54% had no significant abnormality on microbiological testing. The previous literature has emphasised the presence of group B Streptococcus, which we found in only 13% of cases. Other historical triggers included hormone-replacement therapy and recent gynaecological surgery. We did not perform bed-side microscopy but sought to define diagnostic criteria that could be used in the office of a busy practitioner who did not have the time or skills to use a microscope. These were as follows:
History: non-cyclical pain, dyspareunia, itch and discharge
Clinical findings: non-erosive, non-scarring patchy or confluent erythema or petechiae
Exclusion of other causes of chronic vaginitis
Drug and irritant causes excluded by trial of elimination
Microbiology: no pathogens isolated on culture
Treatment response: prompt improvement with intra-vaginal antibiotic (see below)
The aetiology of this condition remains elusive. Some authors believe it is a mild form of lichen planus. We do not agree with this point of view because:
Management
Desquamative inflammatory vulvovaginitis is usually easy to treat. It responds to a number of intra-vaginal antibiotics including clindamycin, mupirocin and metronidazole. We must stress that oral antibiotics are ineffective.
The preparation we use most commonly is 2% clindamycin vaginal cream. Most patients tolerate this; however, in some cases allergy or severe irritation occur. In these patients, metronidazole 0.75% topical preparations may be substituted. Mupirocin 2% is a third option.
Our regimen is:
5 g of clindamycin 2% cream inserted intra-vaginally at night daily for 4 weeks plus
hydrocortisone 1% ointment applied externally twice daily
Almost all patients are asymptomatic and normal to examination by 2–4 weeks of treatment.
Any historical triggers should be modified where possible. If hormone-replacement therapy is implicated, this is ceased at the beginning of the 4 weeks of treatment. If the patient cannot cope without hormone-replacement therapy, it may be restarted after treatment is completed. If the DIV recurs, a decision about the permanent cessation of hormone-replacement therapy will be necessary.
Retreatment or maintenance therapy is often required for DIV. In our study, 95% of patients were symptomatically and objectively improved at initial review. However, 45% of our patients required maintenance therapy. This usually involved application of the antibiotic cream plus hydrocortisone 1% daily externally.
Treatment can be ceased when patients have been asymptomatic for 3 months, but patients should be warned that a relapse might occur. Retreatment invariably results in a rapid response. However, there is a small group of women who had initially presented with a clinical picture of DIV, who subsequently declared themselves as having lichen planus. We conclude from this that DIV may sometimes precede lichen planus. This may have resulted in confusion in the literature, as neither condition is reliably diagnosable by biopsy.
Aphthous Ulceration and Non-Sexually Acquired Genital Ulceration
Aphthous ulceration of the oral mucosa is common, easily recognised and self-limiting. What is not so well known is that aphthae may involve the vulva (Figures 5.4 and 5.5) where they may become a significant problem, not only because they can be very painful but also because the diagnosis can elude clinicians – with unpleasant consequences for patients. The subject of non-sexually acquired genital ulceration, both of the typical recurrent aphthous type and acute form associated with a febrile prodrome, has received scant attention in the medical literature.
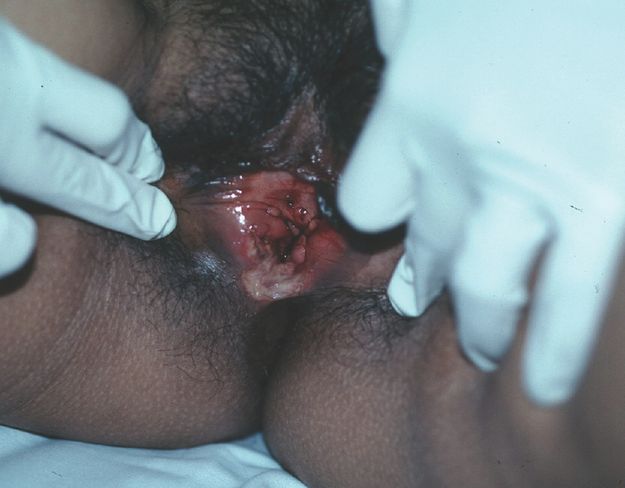
Major aphthous ulcer.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


