Fig. 11.1
Early folliculogenesis . Germ cell cysts break down after birth and primordial follicles form in mouse. In humans, these events occur in utero. Germ cell specific factors considered part of the same pathway are labeled in red. LIF leukemia inhibitory factor, KITL kit ligand, FGF2 fibroblast growth factor, AMH anti-Mullerian hormone, FGF7 fibroblast growth factor 7, NGF nerve growth factor, NT4/5 nerve growth factor receptors, NOBOX newborn ovary homeobox gene, FIGLA factor in the germline alpha, FOXL2 forkhead box L2, GDF9 growth differentiation factor 9, BMP-15 bone morphogenetic protein 15, BDNF neurotrophic factor, NT4/5 neurotrophin 5, FOXO3A forkhead transcription factor, LHX8 LIM homeodomain 8, SOHLH1 spematogenesis and oogenesis helix loop helix 1, SOHLH2 spematogenesis and oogenesis helix loop helix 1
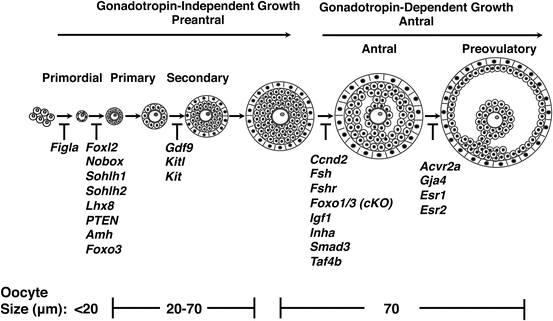
Fig. 11.2
Mouse folliculogenesis and genes that play critical roles. From left to right, postnatal follicle development begins at the primordial stage to the antral stage. Prior to antrum formation, follicle growth is independent of pituitary gonadotropins. Corresponding oocyte diameters in micrometers for each type are also indicated. A subset of genes implicated at various stages of follicular development and their function derived from mouse transgenic models and organ culture experiments is also listed
Primary follicles grow to form larger secondary follicles, which now include a somatic layer of theca cells. Theca cells surround the basal lamina and form two groups of differentiated cells—the theca interna and theca externa. The advanced secondary follicle contains a fully grown oocyte surrounded by a zona pellucida, nine layers of granulosa cells, a basal lamina, a theca interna, and theca externa. Factors critical to advancement to this stage in development include growth differentiation factor 9 and KIT ligand [55, 68]. The development of the antrum, a fluid-filled cavity adjacent to the oocyte, marks the transition of the follicle from being gonadotropin independent to gonadotropin dependent. Granulosa cells undergo mitosis and further differentiation, forming the corona radiata that surrounds the zona pellucida, the corona membrane (which lies interior to the basal lamina), and the cumulus oophorus (which connects the membrane and radiata together). Theca cells also undergo mitosis at this stage and begin to express luteinizing (LH) receptor. Factors critical to this transition include FSH and FSH receptor, Foxo3, insulin growth factor 1, inhibin alpha (inha), G1/S specific cyclin D2 (CCDN2), Smad family member 3 (Smad3), and transcription initiation factor 4B (Taf4b) [60, 69–75]. In response to FSH, antral follicles secrete both estrogen and inhibin, which have a negative feedback effect on FSH production. With the decline of FSH, follicles with fewer FSH receptors undergo atresia, and a dominant follicle that has gained sufficient FSH expression emerges. The transition to the pre-ovulatory follicle relies on estrogen receptors 1 and 2 (Esr1 and Esr2), gap junction protein 4 (Gja4), and activin type receptor 2 (Acvr2) [76–79]. A rise in estradiol from the dominant follicle’s granulosa cells stimulates the LH surge, ultimately leading to oocyte maturation, follicle rupture, and ovulation. This process should remind us that the transcriptional machinery and processes required for mammalian folliculogenesis, unlike what is seen in lower taxa, are not straightforward, and recapitulation of these processes, which demand high levels of cellular energy and an overhaul of cellular machinery, is not insignificant.
Oogonial Stem Cells: Evidence and Pitfalls
The idea that women are born with a finite pool of oocytes has recently been challenged, in the historical midst of many other publications proclaiming the discovery of adult somatic stem cells in virtually every tissue where they have been sought [80]. The challenge has been based on the very imprecise science of follicle counting in mouse ovaries. The numbers of healthy and degenerating (atretic) primordial, primary, and preantral follicles were determined in the ovaries of C57BL/6 mice. This analysis revealed that, despite an increased rate of atresia by the 30th day of life, only approximately one-third of primordial follicles are lost through the life span. What could account for this apparently slower-than-previously appreciated rate of loss? One possibility is a decreased clearance of the atretic follicles. If the total number of oocytes undergoing apoptosis is highest in the newborn and concurrent with new follicle production, this hypothesis could be tested by comparing the total atretic count to the number of healthy-appearing follicles over a brief period shortly after birth [51]. Authors noted that the total atretic count decreased at the end of the observation period, suggesting that many of the degenerating follicles do get cleared. Additionally, given both the rate of observed atresia and the initial postnatal count of healthy follicles, it would be expected that the follicle pool would be completely depleted by young adulthood, which was clearly not the case. Instead, the number of healthy follicles declined by only a small fraction in a period of time where follicle atresia was greatest. The authors also used chemicals such as DMBA and busulfan, known as germ cell toxicants, to buttress the arguments that the low observed oocyte loss in the face of massive atresia has to be balanced by neo-oogenesis. The authors concluded that 77 new primordial follicles were produced daily, an incredibly precise number for a highly imperfect counting methodology. Moreover, the authors claimed that histologic analysis revealed large extrafollicular ovoid cells which were double positive for BrdU and VASA homologue (MVH, a germ cell marker in vertebrates and invertebrates) and were observed in various stages of mitosis following staining with propidium iodide, suggesting that they have a germ cell like proliferative ability [81, 82]. These putative oogonial stem cells (OSCs) were found in the epithelial layer of the ovary, which also stained positive for meiosis-associated gene transcripts, synaptonemal complex protein 3, endonuclease Spo11, and recombinase Dmc1, suggesting that these cells had the capability not only for proliferation but also meiosis. The authors also showed that some oocytes from a GFP positive host can become incorporated into the follicles of GFP negative transplanted ovaries. Taken together, these findings, in the background of the adult stem cell frenzy, were interpreted to mean that oogonial stem cells (OSCs) exist.
A number of concerns were raised by several investigators [49, 83]. First, the scoring of atretic follicles was highly subjective [84]. Second, the mathematical approach taken to calculate the rate of primordial follicle renewal was also criticized because the rate of egress is not constant with age, and more importantly, follicle kinetics vary among mouse strains. The original paper applied the kinetics of a mouse strain with an incredibly high rate of follicle depletion (CBA/Ca) to strain that loses follicles more slowly (C57BL6) [80]. A subsequent modeling study challenged the idea of OSCs [85]. The study was performed to assess the follicle count from day 6 to 12 months of life; the investigators fitted the data to two different models: a fixed pool model and a stem cell model. Only the fixed pool model correctly predicted the observed decline in the primordial follicle pool. Second, the findings of primordial follicle loss following exposure to DMBA don’t mean that oocyte stem cells are eliminated, as this chemical selectively targets primordial follicles [86, 87]. Third, the functional significance of the BrdU/MVH positive ovoid cells noted in the epithelium is unclear. With the limited number of examples provided, the appearance of these cells was consistent with supernumerary germ cells that have been previously noted by confocal microscopy in immature mice and human fetuses [88, 89]. Since young animals were used, these relatively rare cells could very well represent oogonia that were not arrested in meiosis, persisted postnatally, and failed to capture somatic cell support. Most importantly, few, if any, germ cells are found in this location in the adult mammal. The meiotic capability of these cells was also questioned. The presence of various stemness and meiotic markers in rare cells could simply represent false-positive signals, or outlier population of oocytes that was never arrested in meiosis I. Finally, an alternative explanation for the chimeric ovary experiment was offered [90, 91]. Mouse ovaries undergo substantial remodeling with follicle growth and ovulation, and it has been previously shown that even if broken apart, isolated cells and primordial follicles retain the capability of regenerating follicles when transplanted to a recipient animal, possibly through an exchange of somatic and germ cell components and not because of the presence of stem cells [90, 91].
The following year, a claim was made that the origin of oogonial stem cells does not derive from the ovary but from the bone marrow [49]. This was a plausible theory, as multiple papers at the time of the adult stem cell frenzy implicated bone marrow as seeding multiple organs with stem cells [92, 93]. The rationale behind switching the origin of OSCs from ovaries to bone marrow relied on calculations that there are too few OSCs in the ovary to explain its extraordinary ability to produce 77 or so new follicles each day, suggesting an extraovarian source for the growing primordial pool. The bone marrow was suggested because both primordial germ cells and hematopoietic stem cells originate from the same region [94]. Germ cell markers’ (Oct4, Mvh, Dazl, Stella, and Fragilis) as well as the female germ cell specific Nobox expression has been demonstrated in a small subset of the bone marrow cells. The authors then claimed that bone marrow transplants could rescue oocyte production in chemotherapy-sterilized females. This led to the hypothesis that bone marrow derived germ cells could travel through the peripheral blood to the ovary [95].
Again, these findings were met with controversy [84, 96–98]. The main and perhaps most obvious dispute stemmed from the fact that the authors still hadn’t proved that these putative OSCs were capable of ovulation or production of offspring and the group’s findings had yet to be reproduced. They just shifted the origin of these putative OSCs from the ovary to the bone marrow. The presence of germ cell markers in bone marrow is not surprising, as many germ cell markers, both important for male and female gametogenesis, are also expressed in the bone marrow. The chemotherapy-treated controls were not adequately supported through anemia and existing clinical evidence for continued infertility in adult females receiving bone marrow transplants or blood transfusions after ablative chemotherapy for cancer, added to the ongoing controversy.
A definitive experiment put the bone marrow origin of OSCs to rest [99]. Parabiotic mouse models were established to assess whether circulating bone marrow cells contribute to ovulated oocytes. The experiments clearly showed that circulating cells do not contribute to oogenesis. Cells that traveled to the ovary from the bloodstream had characteristics that were in alignment with those of committed white blood cells. The parabiotic setup offered a particularly powerful rejection of the hypothesis that adult bone marrow contributes to ovarian germ cell pool.
In 2009, a new boost to the theory that OSCs originate within the ovary was published. These authors identified a rare population of cells that were stained with the germ cell marker DDX4 and were proliferating, as shown by BrdU incorporation. The DDX4-BrdU positive cells were isolated, cultured, shown to proliferate, and fertilized [100]. These were the first experiments to claim that OSCs give rise to healthy offspring. However, many questions remained. What are the characteristics of these cells that are DDX4-BrdU positive? How rare are they? In our own experiments, we tested for the presence of DDX4-BrdU cells either in wild-type ovaries or ovaries that showed accelerated loss of growing follicles (Fig. 11.3). If OSCs exist, then loss of growing follicles should lead to the enhanced proliferation of OSCs to replenish lost follicles. We did not observe DDX4-BrdU positive cells in either a wild-type or accelerated oocyte loss model. If present, DDX4-BrdU cells are rare and unlikely to play a significant physiologic role. Moreover, the methods for isolating VASA positive cells were met with skepticism because Vasa (DDX4) is known to be a cytoplasmic RNA helicase; second, DDX4 is expressed throughout oogenesis and will not differentiate between different types of oocytes, and third, magnetic sorting does not distinguish between viable and damaged cells, and the isolate may be contaminated with nontargeted cells [101, 102]. A short time later, another group showed that they could use anti-DDX4 antibody and fluorescence activated cell sorting to isolate cells that are stained with germ cell markers and that such cells could be fertilized [103]. However, appropriate controls were lacking. For example, isolated DDX4 positive cells were engineered to ubiquitously express GFP following retroviral transduction, were injected into ovaries, and developing follicles were found to have both GFP positive and negative oocytes. Since antibodies in general have a high degree of non-specificity, it would have been more convincing to perform experiments in parallel with an organ other than the ovary, to make sure that DDX4 antibody wasn’t sorting ovarian somatic stem cells. The presence of GFP positive cells can simply be explained by non-GFP oocytes fusing with GFP cells. Moreover, there was no substantial evidence that these cells go through meiotic stages and there was no evidence that the regenerated oocytes were functional, and the fate of resultant embryos, both genetically and developmentally, was not revealed. If OSCs exist, then alternative scientific approaches should yield results that support existence of OSCs. Several groups have provided evidence to the contrary. Their approaches used sensitive lineage labeling systems to determine whether OSCs exist, and whether they are needed to compensate for follicular losses. The first approach utilized Rosa26rbw+; Ddx4-Cre mice [104] to drive germline specific recombination at the Rainbow cassette [105, 106]. Rosa26rbw+ will label all cells green, but in the presence of Ddx4Cre, the germ cells will fluoresce red, while somatic cells will remain green. Ddx4 positive cells were not found to proliferate or to contribute new oocytes during folliculogenesis. This led to the conclusion that candidate Ddx4 expressing female germline cells are not OSCs. An alternative approach to perform in vivo lineage tracking was also utilized [107]. Germ cells were identified by using an unbiased lineage labeling system in which a general promotor (CAG/chicken b-actin) drives Cre recombinase and estrogen receptor (CreER) in all tissues [108]. Following injection with tamoxifen (tam), excision of a stop cassette within Rosa-Yellow fluorescent protein (ROSA-YFP) occurs, activating YFP expression in the marked cell along with all of its progeny [109]. If OSCs were present, the number of labeled follicles should not decrease and vice versa. The total primordial follicle pool was generally stable, with individual follicles having a half-life of between 10 and 11 months. The lineage tracing experiments provided convincing evidence that OSCs do not exist.
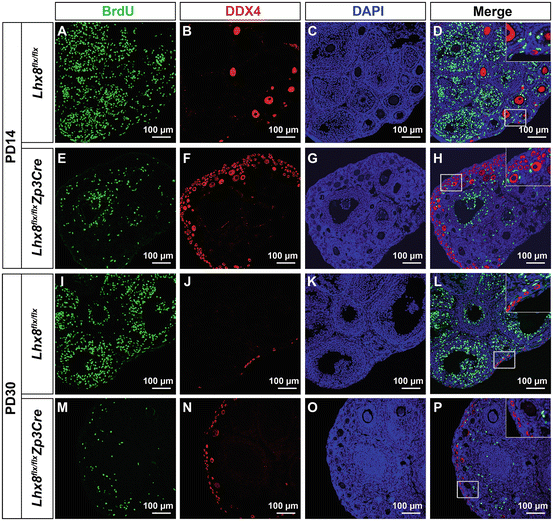

Fig. 11.3
Proliferating germ cells cannot be detected in wild type or Lhx8 flx/flx Zp3Cre ovaries. To detect existence of putative oogonial stem cells, double immunofluorescence staining against DDX4 (germ cell marker) and BrdU (proliferation maker) was performed in postnatal day 14 control (Lhx8 flx/flx ) (A–D), and Lhx8 flx/flx Zp3Cre (E–H), as well as postnatal day 30 control (Lhx8 flx/flx ) (I–L), and Lhx8 flx/flx Zp3Cre (M–P) ovaries. Lhx8 flx/flx Zp3Cre mice eliminate growing follicle pool rapidly, while maintaining primordial follicles. We would expect that elimination of growing follicle pool in Lhx8 flx/flx Zp3Cre mice will stimulate putative oogonial stem cells to proliferate and replenish lost follicles. We didn’t observe DDX4-BrdU positive cells in 10 pairs of ovaries, each examined with 20 independent sections. The insert areas in D, H, L, and P were magnifications of corresponding boxed areas in corresponding panels
Conclusion
Placed in the historical context of the adult stem cell bandwagon that swept biology in the early 2000s, one can clearly see the reasons to believe that the long-standing dogma that women have a finite follicle pool will be overturned. There is little argument that adult somatic stem cells do exist in the ovary. Strong evidence exists that stem like somatic cells reside in the ovarian surface epithelium and serve as precursors to granulosa and other stromal cells [110]. These ovarian stem cells have the capability to undergo clonal expansion following ovulation and have also been found to form the first cohort of follicles in the neonatal ovary [110–113]. However, unlike the evidence for the somatic cells in the ovary, definitive evidence for oogonial stem cells does not exist. The original counting discrepancy that led to the idea of OSCs is flawed. The rarity of DDX4-BrdU cells within the ovary (Fig. 11.3) and the lack of appropriate controls argue that these cells do not contribute significantly to ovarian physiology. The elegant lineage-tracing experiments convincingly contradict the notion that OSCs exist under physiologic conditions.
The ability to generate oocytes de novo has huge potential clinical applications among which are prolonging reproductive life span, replacing iatrogenic ovary loss that may occur during hysterectomy or chemotherapeutic treatments, curing diminished ovarian reserve , and reversing premature ovarian failure. Although the existence and clinical use of oogonial adult stem cells are highly questionable as discussed above, exciting discoveries in the last decade have revealed other promising approaches. In 2006, successful reprogramming of adult human somatic cells into a pluripotent state was accomplished by the transduction of four critical transcription factors, Oct3/4, Sox2, Klf4, and c-Myc [114]. Induced pluripotency has not only opened the door for the generation of patient and disease specific pluripotent stem cells, but it can also be used to correct mutations [115, 116]. Recent studies in mice show that pluripotent cells derived from somatic cells can give rise to oocytes [117]. One can imagine a situation where a young woman with premature ovarian failure could have the genetic mutation that led to her ovarian failure identified, undergo a skin biopsy, convert her cells to a pluripotent state, correct the mutation, and differentiate the pluripotent cells into fertilizable oocytes. Until that day, much work remains to better understand all the quality controls that nature has instituted to produce quality eggs ready for fertilization and new generations.
References
1.
2.
Richards RJ. The tragic sense of life: Ernst Haeckel and the struggle over evolutionary thought. Chicago: University of Chicago Press; 2008.CrossRef
3.
Haeckel E. Natürliche Schöpfungsgeschichte. Berlin: Georg Reimer; 1868. 15th lecture.
4.
Haeckel E. Anthropogenie oder Entwickelungsgeschichte des Menschen. 3rd ed. Leipzig: Wilhelm Engelmann; 1877. p. 144. my translation.
5.
Boveri T. Zur Frage der Entstehung maligner Tumoren. Jena: Gustav Fischer; 1914. p. 2.
6.
Weismann A. Die Continuität des Keimplasma’s als Grundlage einer Theorie der Vererbung. Jena: Gustav Fischer; 1885. p. 5–21.
7.
Satzinger H. Differenz und Vererbung. Geschlechterordnungen in der Genetik und Hormonforschung 1890–1950. Cologne: Böhlau Verlag; 2009. p. 85–97.
8.
Baltzer F. Theodor Boveri: life and work of a great biologist 1862–1915. Berkeley, CA: University of California Press; 1967.
9.
Harwood J. Styles of scientific thought. The German genetics community 1900-1933. Chicago: University of Chicago Press; 1993. p. 52–5.
10.
Haecker V. Über Gedächtnis, Vererbung und Pluripotenz. Jena: Gustav Fischer; 1914. p. 63–85.
11.
Haecker R. Das Leben von Valentin Haecker. Zoologischer Anzeiger. 1965;174:1–22.
12.
Pappenheim A. Zwei Fälle akuter grosslymphozytärer Leukämie. Folia Haematologica. 1907;4:301–8. On Pappenheim and his haematological work see: Dinser, Ricarda. Der Beitrag Artur Pappenheims zur Hämatologie um die Jahrhundertwende. Ruhr-Universität Bochum.
13.
Dantschakoff W. Untersuchungen über die Entwickelung des Blutes und Bindegewebes bei den Vögeln. Anatomische Hefte. 1908;37:471–589. On Dantschakoff’s international career see Satzinger, op. cit. (note 19), p. 231–2, 394–6.CrossRef
14.
Maximow A. Der Lymphozyt als gemeinsame Stammzelle der verschiedenen Blutelemente in der embryonalen Entwicklung und im postfetalen Leben der Säugetiere. Folia Haematologica. 1909;8:125–34. Konstantinov IE. In search of Alexander A. Maximow: the man behin.
15.
Neumann E. Hämatologische Studien I.–III. Virchows Archiv für pathologische Anatomie. 225-77 (1896); 174, 41–78 (1903); 207, 379–412 (1912).
16.
Ehrlich P, Lazarus A. Histology of the blood: normal and pathological. Cambridge: University Press; 1900. p. 81.
17.
Neumann E. Ueber die Bedeutung des Knochenmarkes für die Blutbildung, Vorläufige Mittheilung. Centralblatt für die medicinischen Wissenschaften. 1868; 6(44). Neumann-Redlin v Meding, E. Der Pathologe Ernst Neumann (1834–1918) und sein Beitrag zur Begründung.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


