Respiratory distress syndrome (RDS, HMD)
Bronchopulmonary dysplasia (BPD)
Pneumothorax
Pneumonia/sepsis
Patent ductus arteriosus (PDA)
Necrotizing enterocolitis (NEC)
Retinopathy of prematurity (ROP)
Intraventricular hemorrhage (IVH)
Periventricular leukomalacia (PVL)
Cerebral palsy (CP)
RESPIRATORY DISTRESS SYNDROME
To provide blood gas exchange immediately following delivery, the lungs must rapidly fill with air while being cleared of fluid. Concurrently, pulmonary arterial blood flow must increase remarkably. Some of the fluid is expressed as the chest is compressed during vaginal delivery, and the remainder is absorbed through the pulmonary lymphatics. Sufficient surfactant, synthesized by type II pneumocytes, is essential to stabilize the air-expanded alveoli. It lowers surface tension and thereby prevents lung collapse during expiration (Chap. 7, p. 142). If surfactant is inadequate, hyaline membranes form in the distal bronchioles and alveoli, and RDS develops. Although respiratory insufficiency is generally a disease of preterm neonates, it does develop in term newborns, especially with sepsis or meconium aspiration.
 Clinical Course
Clinical Course
In typical RDS, tachypnea develops, the chest wall retracts, and expiration is accompanied by grunting and nostril flaring. Shunting of blood through nonventilated lung contributes to hypoxemia and metabolic and respiratory acidosis. Poor peripheral circulation and systemic hypotension may be evident. The chest radiograph shows a diffuse reticulogranular infiltrate and an air-filled tracheobronchial tree—air bronchogram.
As discussed further in Chapter 33 (p. 637), respiratory insufficiency can also be caused by sepsis, pneumonia, meconium aspiration, pneumothorax, persistent fetal circulation, heart failure, and malformations involving thoracic structures, such as diaphragmatic hernia. Evidence is also accruing that common mutations in surfactant protein production may cause RDS (Garmany, 2008; Shulenin, 2004).
 Pathology
Pathology
With inadequate surfactant, alveoli are unstable, and low pressures cause collapse at end expiration. Pneumocyte nutrition is compromised by hypoxia and systemic hypotension. Partial persistence of the fetal circulation may lead to pulmonary hypertension and a relative right-to-left shunt. Eventually, alveolar cells undergo ischemic necrosis. When oxygen therapy is initiated, the pulmonary vascular bed dilates, and the shunt reverses. Protein-filled fluid leaks into the alveolar ducts, and the cells lining the ducts slough. Hyaline membranes composed of fibrin-rich protein and cellular debris line the dilated alveoli and terminal bronchioles. The epithelium underlying the membrane becomes necrotic. With hematoxylin-eosin staining, these membranes appear amorphous and eosinophilic, like hyaline cartilage. Because of this, respiratory distress in the newborn is also termed hyaline membrane disease.
 Treatment
Treatment
The most important factor influencing survival is neonatal intensive care. Although hypoxemia prompts supplemental oxygen, excess oxygen can damage the pulmonary epithelium and the retina. However, advances in mechanical ventilation technology have improved neonatal survival rates. For example, continuous positive airway pressure (CPAP) prevents the collapse of unstable alveoli. This allows high inspired-oxygen concentrations to be reduced, thereby minimizing its toxicity. Disadvantages include overstretch of the endothelium and epithelium, which results in barotrauma and impaired venous return (Verbrugge, 1999). High-frequency oscillatory ventilation may reduce the barotrauma risk by using a constant, low-distending pressure and small oscillations to promote alveolar patency. This technique allows optimal lung volume to be maintained and carbon dioxide to be cleared without damaging alveoli. Although mechanical ventilation has undoubtedly improved survival rates, it is also an important factor in the genesis of chronic lung disease—bronchopulmonary dysplasia.
Treatment of the ventilator-dependent neonate with glucocorticoids was used previously to prevent chronic lung disease. The American Academy of Pediatrics now recommends against their use because of limited benefits and increased adverse neuropsychological effects (Watterberg, 2010). Yeh and colleagues (2004) described significantly impaired motor and cognitive function and school performance in exposed neonates. A review by Halliday and associates (2009) supports late postnatal corticosteroid treatment only for infants who could not be weaned from mechanical ventilation. In some studies, inhaled nitric oxide was shown to be associated with improved outcomes for neonates undergoing mechanical ventilation (Ballard, 2006; Kinsella, 2006; Mestan, 2005). Other studies showed no benefits (van Meurs, 2005). Currently, such treatment is considered investigational (Chock, 2009; Stark, 2006).
Surfactant Prophylaxis
Exogenous surfactant products can prevent RDS. They contain biological or animal surfactants such as bovine—Survanta, calf—Infasurf, porcine—Curosurf, or synthetic—Exosurf. Lucinactant—Surfaxin R—is a synthetic form that contains sinulpeptide KL4 to diminish lung inflammation (Zhu, 2008). In a Cochrane review, Pfister and coworkers (2007) found that animal-derived and synthetic surfactants were comparable.
Surfactant therapy has been credited with the largest drop in infant mortality rates observed in 25 years (Jobe, 1993). It has been used for prophylaxis of preterm, at-risk newborns and for rescue of those with established disease. Given together, antenatal corticosteroids and surfactant result in an even greater reduction in the overall death rate. In their review, Seger and Soll (2009) found that infants who received prophylactic surfactant had decreased risks of pneumothorax, pulmonary interstitial emphysema, bronchopulmonary dysplasia, and mortality.
 Complications
Complications
Persistent hyperoxia injures the lung, especially the alveoli and capillaries. High oxygen concentrations given at high pressures can cause bronchopulmonary dysplasia. With this, alveolar and bronchiolar epithelial damage leads to hypoxia, hypercarbia, and chronic oxygen dependence from peribronchial and interstitial fibrosis. According to Baraldi and Filippone (2007), most cases are now seen in infants born before 30 weeks’ gestation and represent a developmental disorder secondary to alveolarization injury. Severe disease and death rates are decreasing, however, long-term pulmonary dysfunction is encountered later. Pulmonary hypertension is another frequent complication. If hyperoxemia is sustained, the infant also is at risk of developing retinopathy of prematurity, formerly called retrolental fibroplasia (p. 656). When any of these develop, the likelihood of subsequent neurosensory impairment is substantively increased (Schmidt, 2003).
 Prevention
Prevention
Antenatal Corticosteroids
The National Institutes of Health (1994, 2000) concluded that a single course of antenatal corticosteroid therapy reduced respiratory distress and intraventricular hemorrhage in preterm infants born between 24 and 34 weeks (p. 657). The American College of Obstetricians and Gynecologists and American Academy of Pediatrics (2012) considers all women at risk for preterm birth in this gestational-age range to be potential candidates for therapy. This is discussed further is Chapter 42 (p. 850). After 34 weeks, approximately 4 percent of infants develop respiratory distress syndrome (Consortium on Safe Labor, 2010).
Amniocentesis for Fetal Lung Maturity
Delivery for fetal indications is necessary when the risks to the fetus from a hostile intrauterine environment are greater than the risk of severe neonatal problems, even if the fetus is preterm. If this degree of risk is absent and the criteria for elective delivery at term are not met, then amniocentesis and amnionic fluid analysis can be used to confirm fetal lung maturity. To accomplish this, there are several methods to determine the relative concentration of surfactant-active phospholipids in amnionic fluid. Fluid acquisition is similar to that described for second-trimester amniocentesis (Chap. 16, p. 297). With this sampling, complications requiring urgent delivery arise in up to 1 percent of procedures (American College of Obstetricians and Gynecologists, 2008). Following analysis, the probability of respiratory distress developing in a given infant depends on the test used and fetal gestational age. Importantly, administration of corticosteroids to induce pulmonary maturation has variable effects on some of these tests.
Lecithin–Sphingomyelin (L/S) Ratio. Although not used nearly as much as in the past, the labor-intensive L/S ratio for many years was the gold-standard test. Dipalmitoylphosphatidylcholine (DPPC), that is, lecithin—along with phosphatidylinositol and especially phosphatidylglycerol (PG)—is an important component of the surface-active layer that prevents alveolar collapse (Chap. 32, p. 624). Before 34 weeks, lecithin and sphingomyelin are present in amnionic fluid in similar concentrations. At 32 to 34 weeks, the concentration of lecithin relative to sphingomyelin begins to rise (Fig. 34-1).
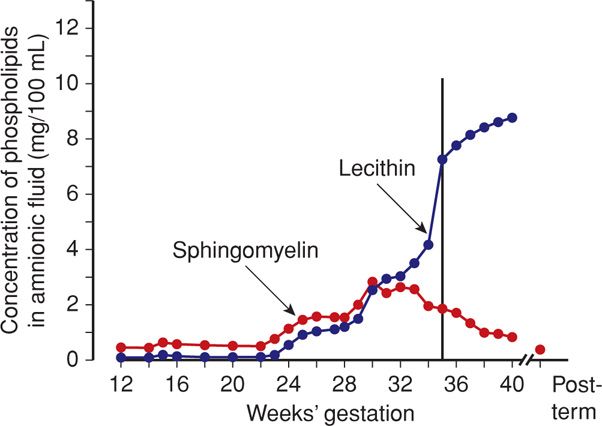
FIGURE 34-1 Changes in mean concentrations of lecithin and sphingomyelin in amnionic fluid during gestation in normal pregnancy. (Redrawn from Gluck, 1973, with permission.)
Gluck (1971) reported that the risk of neonatal respiratory distress is slight whenever the concentration of lecithin is at least twice that of sphingomyelin—the L/S ratio. Conversely, there is increased risk of respiratory distress when this ratio is < 2. Because lecithin and sphingomyelin are found in blood and meconium, contamination with these substances may falsely lower a mature L/S ratio.
Phosphatidylglycerol. Previously, respiratory distress was thought to develop despite an L/S ratio > 2 in infants of women with diabetes. Some recommend that phosphatidylglycerol be documented in amnionic fluid of these women. Based on current evidence, it is unclear if either diabetes, per se, or its level of control causes false-positive phospholipid test results for fetal lung maturity (American College of Obstetricians and Gynecologists, 2008).
Fluorescence Polarization. This automated assay measures the surfactant-to-albumin ratio in uncentrifuged amnionic fluid and gives results in approximately 30 minutes. A ratio of ≥ 50 with the commercially available TDx-FLM test predicted fetal lung maturity in 100 percent of cases (Steinfeld, 1992). Subsequent investigators found the TDx-FLM to be equal or superior to the L/S ratio, foam stability index, or phosphatidylglycerol assessment, including testing in diabetic pregnancies (Eriksen, 1996; Karcher, 2005). The recently modified TDx-FLM II is used by many hospitals as their primary test of pulmonary maturity, with a threshold ratio of 55 mg/g.
Other Tests. The foam stability or shake test depends on the ability of surfactant in amnionic fluid, when mixed appropriately with ethanol, to generate stable foam at the air–liquid interface (Clements, 1972). Problems include errors caused by slight contamination and frequent false-negative test results. Alternatively, the Lumadex-FSI test, fluorescent polarization (microviscometry), and amnionic fluid absorbance at 650-nm wavelength have all been used with variable success. The lamellar body count is a rapid, simple, and accurate method of assessing fetal lung maturity and is comparable to TDx-FLM and L/S ratio (Karcher, 2005).
NECROTIZING ENTEROCOLITIS
This newborn bowel disorder has clinical findings of abdominal distention, ileus, and bloody stools. There is usually radiological evidence of pneumatosis intestinalis—bowel wall gas derived from invading bacteria. Bowel perforation may prompt resection. Necrotizing enterocolitis is seen primarily in low-birthweight newborns but occasionally is encountered in mature neonates. Various hypothesized causes include perinatal hypotension, hypoxia, sepsis, umbilical catheterization, exchange transfusions, and the feeding of cow milk and hypertonic solutions (Kliegman, 1984). All of these can ultimately lead to intestinal ischemia, and reperfusion injury likely also has a role (Czyrko, 1991). The unifying hypothesis for these pathological processes is the uncontrolled inflammatory response to bacterial colonization of the preterm intestine (Grave, 2007).
Treatment for necrotizing enterocolitis is controversial (van Vliet, 2013). A randomized trial of laparotomy versus peritoneal drainage found no difference in survival rates in preterm infants (Moss, 2006). In an observational study, however, Blakely (2006) found that death rates and neurodevelopmental outcomes assessed at 18 to 22 months were more favorable with laparotomy compared with drainage.
RETINOPATHY OF PREMATURITY
Formerly known as retrolental fibroplasia, by 1950, retinopathy of prematurity had become the largest single cause of blindness in this country. After the discovery that the disease resulted from hyperoxemia, its frequency decreased remarkably. The fetal retina vascularizes centrifugally from the optic nerve starting at approximately the fourth month and continues until shortly after birth. During vascularization, excessive oxygen induces severe retinal vasoconstriction with endothelial damage and vessel obliteration, especially in the temporal portion. Neovascularization results, and the new vessels penetrate the retina and extend into the vitreous. Here, they are prone to leak proteins or burst with subsequent hemorrhage. Adhesions then form, which detach the retina.
Precise levels of hyperoxemia that can be sustained without causing retinopathy are not known. Preterm birth results in a “relative” hyperoxia compared with in utero oxygen content even in infants not exposed to higher Fio2. To better understand the oxygen saturation threshold necessary to minimize retinopathy without increasing other adverse outcomes, the National Institute of Child Health and Human Development (NICHD) Neonatal Network performed a randomized trial of oxygenation in 1316 infants born between 24 and 27 weeks (SUPPORT Study Group, 2010). The target range of oxygen saturation was 85 to 89 percent in one arm of this trial and 91 to 95 percent in the other arm. Death before discharge occurred significantly more frequently in the lower-oxygen saturation group—20 versus 16 percent. However, severe retinopathy among survivors developed significantly less often in the lower-oxygen saturation group—8.6 versus 17.9 percent. This study generated considerable controversy as to whether the consent process was adequate for a trial with such end points (Drazen, 2013).
BRAIN DISORDERS
Central nervous system injury in preterm infants usually creates different neuroanatomical sequelae compared with that in term infants (Chap. 33, p. 639). In preterm infants, cerebral lesions detected by neuroimaging include intraventricular hemorrhage, periventricular hemorrhagic infarction, cystic periventricular leukomalacia, and diffuse white matter injury. All of these are strongly associated with adverse neurodevelopmental outcome. Locatelli and colleagues (2010) found a significantly increased incidence of neurological damage in preterm infants who had periventricular hemorrhage, periventricular leukomalacia, or both.
Cranial sonography remains the preferred approach for detecting frequently occurring brain abnormalities and acute events. It is readily available and reliable for detecting common abnormalities and monitoring brain growth. Because cystic injuries may take 2 to 5 weeks to evolve, serial scans are obtained during this time. In infants whose findings are transient and resolve in the neonatal period, prognosis is improved compared with those whose lesions remain and evolve. At the same time, however, between 4 and 10 percent of prematurely born children may develop cerebral palsy in the absence of lesions. Put another way, 90 to 96 percent of preterm infants with cerebral palsy have cerebral lesions that are detectable using cranial sonography.
 Intracranial Hemorrhage
Intracranial Hemorrhage
There are four major categories of intracranial hemorrhage in the neonate (Volpe, 1995). Subdural hemorrhage is usually the result of trauma. Subarachnoid hemorrhage and intracerebellar hemorrhage usually result from trauma in term infants and hypoxia in preterm infants. Periventricular-intraventricular hemorrhage results from either trauma or asphyxia in half of term infants and has no discernible cause in a fourth. In preterm neonates, the pathogenesis of periventricular hemorrhage is multifactorial and includes hypoxic-ischemic events, anatomical factors, coagulopathy, and many others. The prognosis after hemorrhage depends on its location and extent. For example, subdural and subarachnoid hemorrhage often result in minimal, if any, neurological abnormalities. Bleeding into the parenchyma, however, can cause serious permanent damage.
Periventricular–Intraventricular Hemorrhage
When the fragile capillaries in the germinal matrix rupture, there is bleeding into surrounding tissues that may extend into the ventricular system and brain parenchyma. This type of hemorrhage is common in preterm neonates, especially those born before 32 weeks. However, it can also develop at later gestational ages and even in term neonates. Most hemorrhages develop within 72 hours of birth, but they have been observed as late as 24 days (Perlman, 1986). Because intraventricular hemorrhage usually is recognized within 3 days of delivery, its genesis is often erroneously attributed to birth events. It is important to realize that prelabor intraventricular hemorrhage also can occur (Achiron, 1993; Nores, 1996).
Almost half of hemorrhages are clinically silent, and most small germinal matrix hemorrhages and those confined to the cerebral ventricles resolve without impairment (Weindling, 1995). Large lesions can result in hydrocephalus or in degenerated cystic areas termed periventricular leukomalacia (p. 657). Importantly, the extent of periventricular leukomalacia correlates with cerebral palsy risk.
Pathology. Damage to the germinal matrix capillary network predisposes to subsequent extravasation of blood into the surrounding tissue. In preterm infants, this capillary network is especially fragile for several reasons. First, the subependymal germinal matrix provides poor support for the vessels coursing through it. Second, venous anatomy in this region causes stasis and congestion, which makes vessels susceptible to bursting with increased intravascular pressure. Third, vascular autoregulation is impaired before 32 weeks (Matsuda, 2006; Volpe, 1987). Even if extensive hemorrhage or other complications of preterm birth do not cause death, survivors can have major neurodevelopmental handicaps. DeVries and associates (1985) attribute most long-term sequelae of intraventricular-periventricular hemorrhage to periventricular leukomalacia. These degenerated cystic areas develop most commonly as a result of ischemia and least commonly in direct response to hemorrhage.
Incidence and Severity. The incidence of ventricular hemorrhage depends on gestational age at birth. Approximately half of all neonates born before 34 weeks, but only 4 percent of those born at term, will have some evidence of hemorrhage (Hayden, 1985). Very-low-birthweight infants have the earliest onset of hemorrhage, the greatest likelihood of parenchymal tissue involvement, and thus the highest mortality rate (Perlman, 1986). Preterm black infants are at disparate risk for intraventricular hemorrhage (Reddick, 2008).
The severity of intraventricular hemorrhage can be assessed by neuroimaging studies. Papile and coworkers (1978) devised the most widely used grading scheme to quantify the extent of a lesion and estimate prognosis.
Grade I—hemorrhage limited to the germinal matrix
Grade II—intraventricular hemorrhage
Grade III—hemorrhage with ventricular dilatation
Grade IV—parenchymal extension of hemorrhage
Data from the Neonatal Research Network indicate that 30 percent of infants born weighing 501 to 1500 g develop intracranial hemorrhage, and 12 percent are grade III or IV (Fanaroff, 2007). Jakobi and associates (1992) showed that infants with grade I or II intraventricular hemorrhage had a greater than 90-percent survival rate and a 3-percent rate of handicap—similar to control infants without hemorrhage of the same age. The survival rate for infants with grade III or IV hemorrhage, however, was only 50 percent. Extremely-low-birthweight infants with grade I or II hemorrhage have poorer neurodevelopmental outcomes at 20 months than controls (Patra, 2006).
Contributing Factors. Events that predispose to germinal matrix hemorrhage and subsequent periventricular leukomalacia are multifactorial and complex. As noted, the preterm fetus has fragile intracranial blood vessels that make it particularly susceptible. Moreover, preterm birth is frequently associated with infection, which further predisposes to endothelial activation, platelet adherence, and thrombi (Redline, 2008). RDS and mechanical ventilation are commonly associated factors (Sarkar, 2009).
Prevention with Antenatal Corticosteroids. These agents, given at least 24 hours before delivery, appear to prevent or reduce intraventricular hemorrhage incidence and severity. A Consensus Development Conference of the National Institutes of Health (1994) concluded that such therapy reduced rates of mortality, respiratory distress, and intraventricular hemorrhage in preterm infants born between 24 and 32 weeks and that the benefits were additive with those from surfactant therapy. The consensus panel also concluded that benefits of antenatal corticosteroid therapy probably extend to women with preterm premature membrane rupture. A second consensus statement by the National Institutes of Health (2000) recommended that repeated courses of corticosteroids should not be given. They noted that there were insufficient data to prove benefit or to document the safety of multiple courses (Chap. 42, p. 850).
Subsequently, the Maternal-Fetal Medicine Units Network reported that repeated corticosteroid courses were associated with some improved preterm neonatal outcomes, but also with reduced birthweight and increased risk for fetal-growth restriction (Wapner, 2006). Surveillance of this cohort through age 2 to 3 years found that children exposed to repeated—versus single-dose—steroid courses did not differ significantly in physical or neurocognitive measures (Wapner, 2007). It was worrisome, however, that there was a nonsignificant 5.7-fold relative risk of cerebral palsy in infants exposed to multiple steroid courses. At the same time, the 2-year follow-up of the Australasian Collaborative Trial was reported by Crowther (2007). In more than 1100 infants, the incidence of cerebral palsy was almost identical—4.2 versus 4.8 percent—in those given repeated versus single-course steroids, respectively.
Other Preventative Methods. The efficacy of phenobarbital, vitamin K, vitamin E, or indomethacin in diminishing the frequency and severity of intracranial hemorrhage, when administered either to the neonate or to the mother during labor, remains controversial (Chiswick, 1991; Hanigan, 1988; Thorp, 1995). Data from various sources suggest that magnesium sulfate may prevent the sequelae of periventricular hemorrhage, as discussed on page 659.
It is generally agreed that avoiding significant hypoxia both before and after preterm delivery is paramount (Low, 1995). There is presently no convincing evidence, however, that routine cesarean delivery for the preterm fetus presenting cephalic will decrease the incidence of periventricular hemorrhage. Anderson and colleagues (1992) found no significant difference in the overall frequency of hemorrhage in infants whose birthweights were below 1750 g and who were delivered without labor compared with those delivered during latent or active labor. Infants delivered of mothers in active labor, however, tended to have more grade III or IV hemorrhages.
Periventricular Leukomalacia
This pathological description refers to cystic areas deep in brain white matter that develop after hemorrhagic or ischemic infarction. Tissue ischemia leads to regional necrosis. Because brain tissue does not regenerate and the preterm neonate has minimal gliosis, these irreversibly damaged areas appear as echolucent cysts on neuroimaging studies. Generally, they require at least 2 weeks to form but may develop as long as 4 months after the initial insult. Thus, their presence at birth may help to determine the timing of a hemorrhagic event.
 Cerebral Palsy
Cerebral Palsy
This term refers to a group of conditions that are characterized by chronic movement or posture abnormalities that are cerebral in origin, arise early in life, and are nonprogressive (Nelson, 2003). Epilepsy and mental retardation frequently accompany cerebral palsy. The cause(s) of cerebral palsy are different in preterm and term infants (Chap. 33, p. 640).
Cerebral palsy is commonly classified by the type of neurological dysfunction—spastic, dyskinetic, or ataxic—as well as the number and distribution of limbs involved—quadriplegia, diplegia, hemiplegia, or monoplegia. The major types and their frequencies are:
1. Spastic quadriplegia, which has a strong association with mental retardation and seizure disorders—20 percent
2. Diplegia, which is common in preterm or low-birthweight infants—30 percent
3. Hemiplegia—30 percent
4. Choreoathetoid types—15 percent
5. Mixed varieties (Freeman, 1988; Rosen, 1992).
Incidence and Epidemiological Correlates
According to the Centers for Disease Control and Prevention, the prevalence of cerebral palsy in the United States was 3.1 per 1000 children in 2000 (Bhasin, 2006). Importantly, this rate either has remained essentially unchanged or has increased since the 1950s (Torfs, 1990; Winter, 2002). In some countries, the incidence has risen because advances in the care of very preterm infants have improved their survival, but not their neurological prognosis. For example, Moster and associates (2008) presented long-term follow-up of more than 900,000 births in Norway. Of nonanomalous term infants, the cerebral palsy rate was 0.1 percent compared with 9.1 percent in those born at 23 to 27 weeks. Similarly, O’Callaghan and coworkers (2011) studied the epidemiological associations of cerebral palsy and found preterm birth to be the greatest risk factor.
Intraventricular Hemorrhage
Various clinical and pathological data link severe intraventricular hemorrhage—grade III or IV—and resulting periventricular leukomalacia to cerebral palsy. As described earlier, grade I or II hemorrhages usually resolve without extensive tissue injury. Luthy (1987) reported a 16-fold increased risk of cerebral palsy for low-birthweight infants who had grade III or IV hemorrhage compared with the risk in infants who had either no or grade I or II hemorrhage.
Ischemia
Preterm infants are most susceptible to brain ischemia and periventricular leukomalacia. Before 32 weeks, the vascular anatomy of the brain is composed of two systems. One penetrates into the cortex—the ventriculopedal system. The other reaches down to the ventricles, but then curves to flow outward—the ventriculofugal system (Weindling, 1995). There are no vascular anastomoses connecting these two systems. As a result, the area between these systems, through which the pyramidal tracts pass near the lateral cerebral ventricles, is a watershed area vulnerable to ischemia. Vascular insufficiency before 32 weeks leading to ischemia would affect this watershed area first. Resulting damage of the pyramidal tracts may cause spastic diplegia. After 32 weeks, vascular flow shifts toward the cortex. Thus, hypoxic injury after this time primarily damages the cortical region.
Perinatal Infection
Periventricular leukomalacia is more strongly linked to infection and inflammation than to intraventricular hemorrhage. Zupan and colleagues (1996) studied 753 infants born between 24 and 32 weeks, 9 percent of whom developed periventricular leukomalacia. Those born before 28 weeks, those who had inflammatory events during the last days to weeks before delivery, or those who had both were at highest risk. Perlman and associates (1996) found that periventricular leukomalacia was strongly associated with prolonged membrane rupture, chorioamnionitis, and neonatal hypotension. Bailis and coworkers (2008) reported that chronic—and not acute—placental inflammation was associated with leukomalacia.
Fetal infection may be the key element in the pathway between preterm birth and cerebral palsy (Burd, 2012; Leviton, 2010). In the pathway proposed in Figure 34-2, antenatal reproductive tract infection evokes the production of cytokines such as tumor necrosis factor and interleukins-1, -6, and -8. These in turn stimulate prostaglandin production and preterm labor (Chap. 42, p. 838). Preterm intracranial blood vessels are susceptible to rupture and damage, and the cytokines that stimulate preterm labor also have direct toxic effects on oligodendrocytes and myelin. Vessel rupture, tissue hypoxia, and cytokine-mediated damage result in massive neuronal cell death. Glutamate is released, stimulating membrane receptors to allow excess calcium to enter the neurons. High intracellular calcium levels are toxic to white matter, and glutamate may be directly toxic to oligodendrocytes (Oka, 1993).
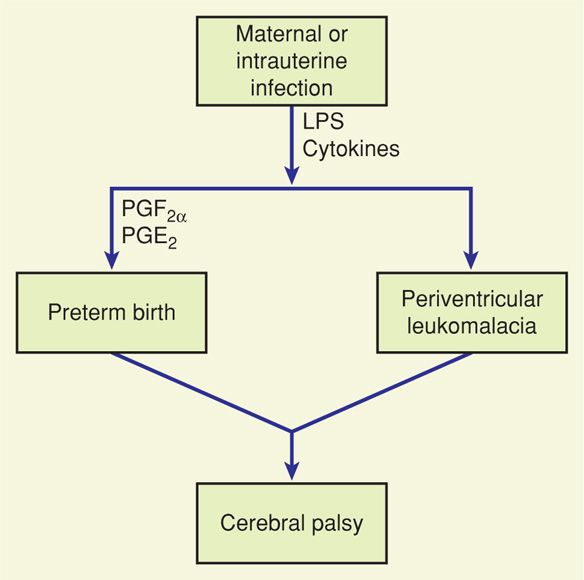
FIGURE 34-2 Schematic representation of the hypothesized pathway between maternal or intrauterine infection and preterm birth or periventricular leukomalacia. Both potentially lead to cerebral palsy. LPS = lipopolysaccharide; PG = prostaglandin.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


