Fig. 4.1
CT of the abdomen without administration of oral contrast in a fasting 2-year-old child in supine position. CT shown in axial (A) plane. Note fluid (labeled “F”) and air (Labeled “A”) in distended stomach. Measured volume of fluid in stomach was 41.8 mL (3.3 mL/kg). Courtesy of Mohamed Mahmoud, MD
There is a presumption that the relative risk of aspiration is lower during sedation than under general anesthesia, and that protective airway reflexes are retained fully during sedation. It is important to note that the progression from mild sedation or analgesia to general anesthesia represents a continuum not easily divided into discrete stages [4]. Anyone receiving moderate or deep sedation should be treated similarly to those receiving general anesthesia because the sedation level can change rapidly and deepen subtly with subsequent impairment of airway reflexes.
Although aspiration is a widely feared complication of general anesthesia, fortunately clinically relevant aspiration in modern anesthesia practice is exceptionally rare in pediatrics. The incidence is estimated to be 1 in 10,000 to 10 in 10,000, with the wide reported range likely due to variation in research methodologies, definitions, and reporting sensitivities [5]. In those undergoing general anesthesia, approximately two-thirds of aspiration occurs during manipulation of the airway (endotracheal tube placement and removal) [6]. The multicenter Pediatric Sedation Research Consortium collected data on 49,836 propofol sedations in children: Aspiration during sedation occurred four times (0.04 %) [7]. A retrospective study by Sanborn et al. of 16,467 sedations during imaging procedures in children using chloral hydrate, midazolam, fentanyl, or pentobarbital found 70 (0.4 %) respiratory incidents; only two patients of 16,467 aspirated (0.012 %) [8].
The low incidence of aspiration pneumonia with sedation and anesthesia may be attributed to the fact that the stomach is very distensible and can accommodate a large volume before resting intragastric pressure rises [9]. Intragastric pressure must exceed the barrier pressure of the lower esophageal sphincter (LES) for regurgitation to occur. The barrier pressure of the LES does not appear to be as easily overcome under general anesthesia as is widely believed [9].
The American Society of Anesthesiology’s (ASA) Task Force on Fasting has published consensus guidelines for elective anesthesia: clear fluids, 2 h; breast milk, 4 h; formula, 6 h; and solids, 8 h [10]. These guidelines are intended for healthy patients of all ages undergoing elective procedures; they are not intended for patients with coexisting diseases or conditions that may delay gastric emptying such as diabetes, hiatal hernia, gastroesophageal reflux, or bowel obstruction. The ASA acknowledges that there is insufficient evidence to codify preoperative fasting times. In addition, the task force does not offer specific guidance for fasting times for emergency procedures.
When practitioners formulate a plan for sedation for emergency procedures in children who have not fasted, the risks of sedation and the possibility of aspiration must be balanced against the benefits of performing the procedure emergently. The American College of Emergency Physicians (ACEP) Clinical Policy on Sedation assesses risk based on the nature of last oral intake and the urgency of the procedure (Table 4.1) [11]. In this setting, aspiration has been found to be very rare among patients sedated in an emergency room setting for procedures, regardless of fasting status [12].
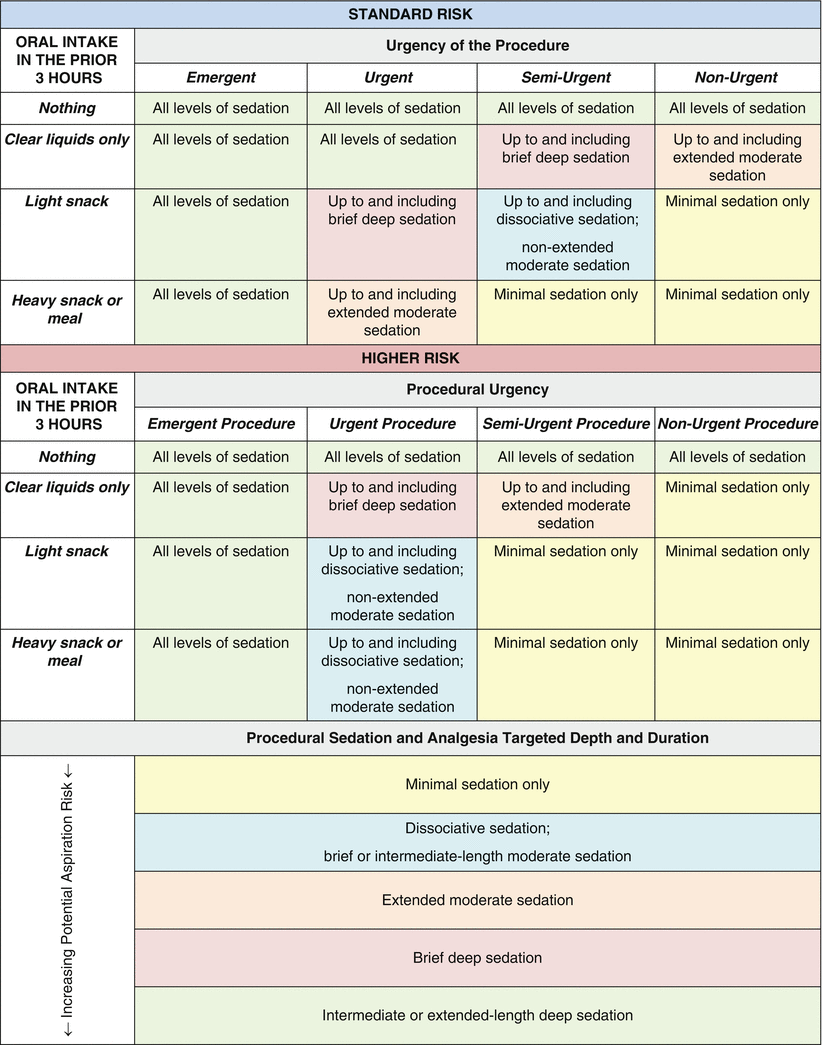
Table 4.1
Prudent limits of targeted depth of ED procedural sedation

There is an ongoing debate regarding the administration of oral contrast for Computerized Tomography (CT) prior to sedation. The administration of oral contrast less than 2 h before sedation-anesthesia is at odds with elective NPO guidelines, and in theory would increase the risk of aspiration pneumonia. Sedation practitioners are asked to make an exception to the fasting guidelines and permit the use of enteric contrast material with CT in order to obtain an accurate study. There does not appear to be a perfect resolution to this issue, since waiting several hours after administration of contrast often results in inadequate opacification of the small bowel and a poor study [13].
Small bowel transit time can be as rapid as 15 min and on average is 1 h 24 min [14]. In one study, in 83 % of cases small bowel transit time was less than 2 h [14]. Inadequate opacification of the small bowel can lead to lack of distinction between small bowel loops and fluid collections or masses [13].
At one author’s institution, administration of contrast begins 2 h before and ends 1 h prior to anesthesia-sedation. The challenge lies in balancing technical factors governing the image quality of the study with safety concerns related to sedating a child with a potentially full stomach for an elective CT. A recent retrospective chart review concluded that administering oral contrast material within 2 h of propofol sedation for abdominal CT in children appears to be relatively safe. The data sample, however, was small relative to the reported incidence of aspiration in the literature [15]. Currently we are not aware of any clear consensus among institutions that care for these patients. Some clinicians may choose to perform rapid sequence induction of general anesthesia with endotracheal intubation while others may choose deep sedation without definitive airway protection. Others may negotiate with radiologists to have the oral contrast given 2 h before the study or administered through an oral gastric tube after placement of an endotracheal tube [16, 17].
When Not to Proceed
Barring emergent or life-threatening circumstances, situations arise in which—despite pressure from consultants, providers, and/or families—the practitioner should forgo sedation outside of the operating room for a more opportune time, setting, or facility. Proper monitoring, rescue equipment, and sufficient staff should be in place. The provider should use sound clinical judgment before proceeding, informed by the patient’s risk for complications and the urgency of the procedure, as well as practical concerns such as the ability to dedicate the necessary time, attention, and human resources to the endeavor. The following section is a broad overview that will address specific safety considerations and focused assessments in important special populations.
Preparation for and Considerations in Special Populations
Asthma and Reactive Airway Disease
The child who wheezes presents a common challenge to the sedation practitioner. Transient wheezers are infants whose symptoms are provoked by an active viral respiratory infection. These children typically “outgrow” their reactivity within the first few years of life. After the toddler and preschool period, non-atopic wheezers continue to experience wheezing with active viral illnesses, but are not likely to develop lifelong symptoms. Both transient and non-atopic wheezers tend to have mild reactions to the inciting event. Atopic wheezers are equally sensitive to viral illnesses, but often also suffer from allergy, allergic rhinitis, and atopic dermatitis. These children are at highest risk for severe and persistent symptoms exacerbated by a variety of infectious and/or environmental factors [18].
The diagnosis of asthma is difficult to make under the age of 6, since there is significant overlap with reactive airway disease and pulmonary function tests are problematic in young children. In those with an established diagnosis of asthma, the assessment of symptoms follows a step-wise approach (Table 4.2).
Table 4.2
Asthma severity assessment in children older than 5 years of age
Clinical features | Mild intermittent asthma | Mild persistent asthma | Moderate persistent asthma | Severe persistent asthma |
|---|---|---|---|---|
A. Symptoms: wheezing, coughing, chest tightness | Symptoms ≤2 times/week | Symptoms >2 times/week but <1 time/day | Daily symptoms | Continual symptoms |
Asymptomatic between brief exacerbations | Exacerbations 2 or more time/week; may last days | Frequent exacerbations | ||
B. Activity limitations | No activity limitations | Activity may cause exacerbations | Activity causes exacerbations | Limited physical activity |
C. Nocturnal symptoms | ≤2 times/month | >2 times/month | >1 time/week | Frequent nighttime symptoms |
D. Lung function | PEF or FEV1 ≥ 80 % of predicted or personal best | PEF or FEV1 ≥ 80 % of predicted or personal best | PEF or FEV1 > 60 % and <80 % of predicted or personal best | PEF or FEV1 ≤ 60 % of predicted or personal best |
In addition to the assessment of severity of symptoms, confirm the overall control of symptoms and what level of therapy the child is currently receiving. It is also helpful to ascertain the responsiveness that the child has shown to previous exacerbations [19]. This is especially important in the planning of procedures that involve airway stimulation or those that would require frequent suctioning.
Children with a history of either reactive airway disease or diagnosed asthma are at risk for bronchial hyperreactivity (40 % of school-aged children with asthma) [20]. Bronchial hyperreactivity may persist for weeks after an exacerbation. For this reason, a careful history of recent illness, changes in medication, and history of hospitalization are important in all children with a history of wheezing. In general in children with stable and controlled asthma or reactive airway disease, the peri-procedural risk for bronchospasm is low and is not associated with a significant morbidity [21].
A recent prospective study found that patient factors (readily known on pre-procedural assessment) such as active respiratory symptoms, eczema, family history of asthma, rhinitis, or exposure to tobacco smoke were associated with an increased relative risk of peri-procedural respiratory adverse events such as airway obstruction, oxygen desaturation (<95 %), and severe or sustained cough [22]. In patients with active symptoms, the practitioner should determine the severity of illness and weigh this with the urgency and importance of the procedure. The actively wheezing patient should have his current illness addressed immediately, and if the procedure is to go forward, a plan for pre-, intra-, and post-procedure treatment should be formulated to anticipate and manage potential complications such as bronchospasm.
Autism, Developmental Delay, and Intellectual Disability
Autism spectrum disorders (ASD) are characterized by neurodevelopmental impairments in three major domains: behavior, communication, and socialization [23]. Although the rate of diagnosis of ASD has markedly increased recently, its pathogenesis is incompletely understood; the current consensus is that autism has a genetic basis with possible contributing environmental factors. Approximately 40–62 % of children with ASD demonstrate some learning disability [24].
Children with intellectual disability, developmental delay, or ASDs require a holistic view in preparation for sedation. Caretakers are typically very helpful in sharing the child’s past reactions to the procedure, and may be vocal in their preferences in the timing, type, and route of administration of sedatives. The practitioner would do well to consider the caregivers’ experience with their child and weigh this with the practicalities and requirements of the procedure at hand.
These children may exhibit challenging behavior, especially when anxious or stressed, such as punching/slapping/pulling (50 %) or kicking (24 %) [25]. Boys and adolescent males form the majority (66 %) of children with challenging behavior [26]. These behaviors may be exacerbated by frequent and sometimes unpleasant interactions with the health care system. Observing the child while non-stressed during the pre-sedation assessment may help to reveal caregiver-patient dynamics as well as to inform the clinician of how best to keep him calm and cooperative. Non-pharmacologic methods such as distraction, storytelling, watching videos, or playing games are particularly helpful in this setting and during the induction/pre-procedural period. (Refer to Chap. 34.)
Intellectual, developmental, and learning disabilities are not a specific medical condition, but rather manifestations of neurologic disease. It is important to note that comorbidities are common, such as epilepsy (44 %), psychiatric disorders (50 %), and gastroesophageal reflux (49 %) [24]. The pre-procedural assessment should include a review of medical conditions, frequency and control of symptoms, and current medications.
A small observational study found that as a group, children with developmental delay (given the prevalence of substantial neurologic comorbidities) may have a smaller airway diameter at the level of the soft palate when sedated for magnetic resonance imaging (MRI). The authors’ findings were thought to be multifactorial: anatomic (different airway shape), physiologic (abnormal airway tone), and pharmacologic (increased susceptibility to sedative) [27]. In this light, concurrent illness such as viral respiratory symptoms should be considered carefully in these patients.
If the child requires pretreatment, one may start with noninvasive approaches such as the oral route for pre-sedation, the intranasal route to facilitate IV access if needed, and the intramuscular route if necessary. Nitrous oxide, if available, may be a good choice if the child sees the device as a novelty or game, rather than as a restraint. Close attention to risk factors for pre-procedural anxiety or behavioral challenges is important, as these are associated with post-procedural delirium and maladaptive behaviors, which complicate the feasibility of a successful outpatient visit [28].
Anticipating behavioral disruptions and having a ready plan for escalation of treatment are essential. Discussion with the caregiver before the procedure may help to decrease his or her anxiety, allowing for a capable, present, and calm ally in the endeavor. This includes the timing and threshold for physical restraint if needed, based on the urgency and nature of the procedure. A brief pre-sedation “team huddle” with caregivers and staff to review the sedation plan may promote a smooth procedure and help to avoid injury to the patient, parents, practitioner, or staff.
Bronchopulmonary Dysplasia
Bronchopulmonary dysplasia (BPD) is the most common cause of chronic lung disease in infants. It affects premature infants who survive the acute phase of respiratory distress syndrome and is characterized by the need for supplemental oxygen beyond 4 weeks of life. BPD is thought to develop after prolonged periods of mechanical ventilation and exposure to high concentrations of inspired oxygen. Other proposed pathophysiologic mechanisms include initial volume overload, increased pulmonary blood flow, and generalized inflammation. These patients typically have decreased lung compliance, airway hyperactivity, lung hyperinflation, rapid respiration, wheezing, cough, and frequent episodes of fever, desaturation, hypercarbia, abnormal functional airway growth, and increased risk for bradycardia and congestive heart failure (CHF) [29].
Implications of BPD in sedation-anesthesia include tracheomalacia, tracheal granuloma, subglottic stenosis, increased airway reactivity and bronchospasm, and diuretic-induced electrolyte disorders. Adequate pre-procedure preparation should focus on optimizing oxygenation, reducing airway hyperactivity, and correcting electrolyte abnormalities. These children require special attention to fluid balance with careful titration of fluids during the procedure. A laryngeal mask airway (LMA) is less irritating to both the upper and lower airways; it may offer some advantage in reducing the incidence of post-procedural coughing, wheezing, and hoarseness compared to endotracheal intubation in these patients.
Cerebral Palsy
Cerebral palsy (CP), a nonprogressive, permanent disorder of motor function and posture, is the most common physical disability in childhood, occurring in 2–2.5 in 1,000 births [30]. The majority of cases are of unknown etiology. Known associations are multifactorial: prematurity (78 %), intrauterine growth restriction (34 %), intrauterine infection (28 %), antepartum hemorrhage (27 %), and maternal alcohol use (threefold increased risk) [31, 32]. One in four have epilepsy and one in five have a sleep disorder [33].
The spectrum of disease varies from mild focal weakness with normal intelligence to total body spasticity and severe intellectual disability. CP may be classified by the predominant motor component: spasticity, ataxia, or dyskinesia [34]. Medical therapy emphasizes control of spasticity with medications, injections, or surgery. In the pre-sedation assessment, the type, dosage, and route of medications are important especially if there will be prolonged fasting. The clinician should determine the presence (and recent setting changes) of an intrathecal pump. Although rarely an issue, children with recent Botulinum toxin type A injection (for local control of spasticity) if unwittingly overdosed may later experience relative respiratory muscle weakness, which may be exacerbated during sedation [35].
Common comorbidities such as scoliosis, gastroesophageal reflux, decubitus ulcers, and skin infections should be assessed for control of disease. This will help in planning for successful positioning (to optimize ventilation and comfort), IV access, and ready access to the airway if advanced measures are needed during the procedure. Children with CP often have considerable drooling due to difficulty in swallowing secretions; plan for frequent suctioning. Atropine or glycopyrrolate may be considered for their anti-sialagogue effect, but they may also thicken lung secretions and potentially increase the risk of lung infection in CP patients [34].
Part of the pre-sedation assessment is anticipating and avoiding pitfalls in the care of children with CP. Chronic low fluid intake and relative malnutrition put the child at risk for pre-renal failure and the development of pressure ulcers. Careful attention to fluid replacement (especially during prolonged fasting periods) and proper positioning of the patient during the procedure will help to attenuate these risks. Other common challenges are the presence of extremity casts that may obscure blood loss (from trauma or the procedure itself) or developing compartment syndrome from malpositioning.
Pain control in intellectually disabled children is an important issue. Clinician understanding of the analgesic needs of these children is changing, and there is evidence to suggest that they may, in fact, be more sensitive to pain than non-disabled children [36]. Unfortunately, these vulnerable children are often undertreated due to barriers in communication or misinterpretation of behaviors [37]. Children on chronic opioids may have 30–100 % higher dosage requirements than opioid-naïve children [38]. Control of symptoms should begin early in the visit to promote a successful procedure and post-procedure course.
Congenital Heart Disease
Congenital heart disease (CHD) occurs in approximately 8 in 1,000 live births [39]. The most common acyanotic lesion is a ventricular septal defect; the most common cyanotic lesion is the tetralogy of Fallot. Although lesions may be classified as acyanotic or cyanotic and/or ductal dependent or not, the clinician may risk stratify based on whether the child has been fully repaired or whether his lesion involves palliation. That is, a child with a repaired ventricular septal defect and normal baseline oxygenation may have no long-term sequelae relevant to sedation while a child with single ventricle pathology, a palliative shunt (e.g., hypoplastic left heart syndrome status-post Fontan procedure), or baseline low oxygen saturation requires a more judicious approach.
Children with cyanotic disease with or without palliative surgery are very sensitive to changes in volume status, as many are pre-load dependent. In addition, certain lesions are more prone to dysrhythmias [40]. Their low baseline oxygen saturations offer little to no reserve in times of stress. For this reason and in general, children with cyanotic heart disease are poor non-emergent outpatient candidates for sedation beyond mild anxiolysis [40–42].
Although each lesion has a unique set of considerations in the pre-sedation assessment, current functional status is most informative of appropriateness for sedation outside of the operating room. Children with CHD (both cyanotic and acyanotic lesions) often develop some degree of CHF. The New York Heart Association (NYHA) classification was originally designed for adults, and is often applied to children (Table 4.3) [41]. The Ross classification was designed specifically for children and mirrors the NYHA classification [43]; recently a detailed age-specific modification to the Ross classification has been proposed [44].
Class | NYHA classification | Ross classification |
|---|---|---|
I | No symptoms | No limitations or symptoms |
II | Symptoms with moderate exertion | Mild tachypnea or diaphoresis with feeding in infants; dyspnea on exertion in older children |
III | Symptoms with mild exertion | Marked tachypnea or diaphoresis with feeding or exertion |
IV | Symptoms at rest | Symptomatic at rest with tachypnea, retractions, grunting, or diaphoresis |
Both the NYHA and the Ross classifications assess current symptoms; neither discriminates well in the early stages of disease. Since overt heart failure symptoms are a late sign in children (due to compensatory mechanisms), and the sedating clinician is interested in detecting subtle risk factors, an updated heart failure staging classification has been proposed (Table 4.4).
Stage | Interpretation |
|---|---|
A | Increased risk of developing heart failure, but with normal cardiac function and size |
B | Abnormal cardiac morphology or function, with no heart failure symptoms or history of symptoms in the past |
C | Underlying structural or functional heart disease and heart failure symptoms past or present |
D | End-stage heart failure |
Stages A and B correspond to NYHA I, and stage C corresponds to NYHA II and III. Stage D patients typically require inotropic and/or ventilator support. In addition to the above, the assessment should include the child’s general health and change in behavior, oral intake, or urine output. A recent cough or taking longer to feed may be subtle alerts to hypervolemia and poor control of CHF. On examination, infants may be in mild to moderate respiratory distress and/or have evidence of hepatic engorgement, a sign of right-sided heart failure (N.B. peripheral edema as seen in adults in CHF is rare in children).
Recent illnesses, especially upper respiratory tract infections (URIs), are especially important to note in these children, as airway reactivity and changes in pulmonary vascular resistance are not well tolerated in children with CHD. A thorough review of previous surgeries and complications, current medications, and drug allergies is required. Anticoagulants may need to be held for the procedure in consultation with the child’s cardiologist. The presence of an implantable cardiac defibrillator or pacer should be determined and recent changes or complications noted [47].
Prophylaxis for bacterial endocarditis is recommended for all dental procedures only in children with high-risk historical features (Table 4.5). In eligible children, it is reasonable to give prophylaxis for procedures on the respiratory tract, infected skin, or musculoskeletal tissue. Prophylaxis is no longer recommended for gastrointestinal or genitourinary procedures.
Table 4.5
Cardiac conditions associated with the highest risk of adverse outcome from endocarditis for which prophylaxis with dental procedures is reasonable [48]
Prosthetic cardiac valve or prosthetic material used for cardiac valve repair |
Previous infectious endocarditis |
Unrepaired cyanotic CHD, including palliative shunts and conduits |
Completely repaired congenital heart defect with prosthetic material or device, whether placed by surgery or by catheter intervention, during the first 6 months after the procedure |
Repaired CHD with residual defects at the site or adjacent to the site of a prosthetic patch or prosthetic device (which inhibit endothelialization) |
Cardiac transplantation recipients who develop cardiac valvulopathy |
Cystic Fibrosis
Cystic fibrosis (CF) is the most common fatal inherited disease in Caucasians, and exists in smaller frequencies in other racial groups [49]. The basis of its pathophysiology is a mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) protein, a chloride channel found in all exocrine tissues. As such, CF is a multi-organ system disease, involving impaired lung function, pancreatic insufficiency and diabetes mellitus, hepatobiliary disease and cirrhosis, bone disease, and genitourinary disease. Pulmonary complications account for over 90 % of the morbidity and mortality in CF patients [50].
CF demonstrates a spectrum not only in terms of organ systems involved but also in severity of disease burden in the individual patent [51]. For this reason, the pre-sedation assessment should include pointed questioning about the child’s frequency of illness, strength of cough, amount of sputum produced, airway reactivity, and history of recovery from procedures and illnesses. A thorough review of current therapies and recent acceleration of treatment may reveal the child’s current trajectory of disease.
Younger children with CF have more reactive airways, which may respond to β(beta)-agonists. It is important to note, however, that older children may have worsening expiratory airflow with the use of bronchodilators. This is due to progressive damage to cartilaginous support in the lower airways; bronchial muscle hypertrophy may in fact help to “stent” the airways open [52]. In these patients, bronchodilators may result in “floppy” lower airways, and impaired gas exchange. A careful history regarding response to β(beta)-agonists is important to anticipate and avoid intra-procedure complications.
In addition to acute exacerbations and worsening lung infections, children with CF are at risk for apical blebs (up to 3.4 %) that may cause spontaneous pneumothorax [50]. Planning for sedation of a child with CF should include preparation for the management of this complication, such as oxygen therapy, IV catheters for decompressive thoracostomy, and a plan for emergent definitive chest tube thoracostomy. Chronic lung disease may manifest in chronic hypoxia and hypercarbia with resulting increases in pulmonary vascular resistance and pulmonary hypertension. An electrocardiogram (ECG) with evidence of cor pulmonale is an ominous sign [53].
Control of diabetes mellitus, if present, should be addressed. The presence of liver disease should be noted, as hepatic clearance of medications may be enhanced in early disease and impaired with the onset of cirrhosis; liver function tests are unreliable in this context [54]. Older CF patients may develop distal intestinal obstruction syndrome (DIOS) in the colon and ileum, mimicking medical and surgical causes of nausea, vomiting, abdominal pain, and distention [55]. Volume depletion, chronic narcotics, and medication nonadherence put the patient at higher risk [50].
If possible, a review of the medications given during previous procedures may be helpful in planning for sedation. Patients with CF may have higher opioid and benzodiazepine requirements than patients without CF [56]. Plan to balance titrating to effect with possible impairment of overall oxygenation and ventilation during the procedure.
Diabetes Mellitus
Type 1 (insulin-dependent) diabetes mellitus (DM) accounts for over 90 % of DM cases in children [57]. Early onset of type 2 (non-insulin-dependent) DM is rising with obesity rates in children [57]. Other less common causes of DM in children include maturity onset diabetes of youth (MODY), insulin resistance syndromes (idiopathic), genetic syndromes (chromosomal abnormalities, congenital disorders of the pancreas), and secondary diabetes (e.g., drugs such as corticosteroids) [58].
The clinician should gain a general view of the patient’s overall diabetes control and any recent change in regimen. A thorough account of the child’s medications (e.g., insulin, oral hypoglycemic sulfonylureas, oral biguanide) and timing of the last dose should be reviewed. Patients may have taken a recent dose of medication, only to be unexpectedly fasting during the visit. Physical exam should pay close attention to volume status, as these children are at risk for hypovolemia. If an insulin pump is found, the silastic catheter may be removed before the procedure to ensure that ongoing insulin is not administered to the fasting child. A baseline fingerstick blood glucose will be helpful in the initial assessment.
Regardless of the type or current control of the patient’s diabetes, the overall goal during sedation is to avoid hypoglycemia and excessive hyperglycemia [58, 59]. When appropriate, IV fluids may be given, and if the procedure is prolonged, supplemental glucose with frequent fingerstick blood glucose monitoring. Case reports demonstrate the importance of glucose monitoring in DM patients undergoing sedation: hypoglycemic coma may be confused for deep or prolonged sedation [60].
Endocrinopathies
Knowledge of the normal anatomy and physiology of the endocrine glands is essential in understanding their potential pathophysiologic effects relevant to procedural sedation. In this section we will outline the considerations for sedating a child with adrenal insufficiency, hypothyroidism, hyperthyroidism, or diabetes insipidus (DI).
The adrenal cortex synthesizes and secretes steroid hormones (glucocorticoids, mineralocorticoids, and sex steroids) that are essential to life. Glucocorticoids (especially cortisol) play a critical role in the body’s response to stress and play an important role in maintaining vascular tone. Causes of adrenal insufficiency can be classified as primary (adrenal gland dysfunction), secondary (the pituitary gland dysfunction), or tertiary (hypothalamic dysfunction). The most common cause of adrenal insufficiency is long-term administration of exogenous glucocorticoids via oral, intravenous, inhaled, intranasal, or topical routes. Even a short course (5 days) of prednisone mildly suppresses the hypothalamic–pituitary–adrenal axis for 5 days after discontinuation (usually without clinical sequelae in the healthy patient). Long-term glucocorticoid use produces adrenal cortical atrophy as a result of chronic suppression of ACTH production, requiring variable recovery times of up to 1 year [61].
The practice of providing perioperative glucocorticoid replacement therapy to patients with adrenal insufficiency is well established. Insufficient levels of cortisol can be produced in response to stress in these patients, posing the risk of acute adrenal crisis with hypotension and cardiovascular collapse.
Peri-procedural stress dosing depends on the duration and invasiveness of the procedure. Most elective minor procedures and noninvasive diagnostic studies do not warrant supplementation with additional glucocorticoids. A continuation of the current dose of corticosteroids is sufficient to maintain cardiovascular function in patients who receive long-term administration of exogenous glucocorticoids [62]. It is extremely important to note that primary hypopituitarism is a condition that always requires peri-procedure steroid supplementation regardless of the daily dose taken. Parenteral cortisol (e.g., Solu-Cortef) at a dose of 0.5–1 mg/kg every 6 h is recommended for perioperative, intensive care, or emergency department indications for up to 72 h [63].
Thyroid hormones are integral to the normal physiology of every organ system of the human body, playing a crucial role in regulating myocardial function, pulmonary ventilation, energy homeostasis, vascular tone, water and electrolyte balance, and normal function of the central nervous system. The most important adverse effects of hypothyroidism include impaired cardiac contractility with decreased cardiac output, increased peripheral vascular resistance, and decreased blood volume and peripheral oxygen consumption.
A detailed history should be obtained from the patient or the family about prior thyroid disease, thyroid surgery, radiation therapy (radioactive iodine or neck irradiation), treatment with any thyroid medications, or family history of thyroid disease. Physical examination is equally important. Dry skin, a slowed deep tendon reflex relaxation phase, bradycardia, and hypothermia are all signs of clinical hypothyroidism. Children with known hypothyroidism have increased sensitivity to anesthetic-sedative agents; these children should have documented normal thyroid function tests before elective procedures.
Hyperthyroidism is less common in children than hypothyroidism and is most commonly caused by Graves disease. The classical features of thyrotoxicosis include hyperactivity, weight loss, tremor, heat intolerance, dyspnea, insomnia, diarrhea, and nervousness. Cardiovascular effects of hyperthyroidism include palpitations, tachycardia, atrial fibrillation, and congestive cardiac failure. Thyroid storm can be lethal. Fortunately, it is rarely seen due to widespread use of antithyroid drugs. In an attempt to prevent this catastrophic complication, these children should be euthyroid before the procedure. Thyroid storm responds to symptomatic treatment including parenteral β(beta)-blockers and propylthiouracil.
The clearance and distribution volume of propofol are increased in hyperthyroid patients. When total intravenous anesthesia is used, propofol infusion rates should be increased to reach anesthetic blood concentrations [64].
Optimal anesthetic-sedative care of patients with history of DI requires an understanding of the complex pathophysiology of this disease. Arginine vasopressin (AVP) is produced within the hypothalamus, and it is normally stored for release in the posterior pituitary gland. After its release, AVP acts on V2 receptors in the collecting tubules of the nephron in order to allow for effective urine concentration.
DI is a syndrome manifested by high output urine, low urine specific gravity (<1.005), high plasma osmolality (>200 mOsm/L), and high plasma sodium (>150 mEq/L). Nephrogenic DI occurs when the kidney is unable to control plasma osmolality due to a defect in the action of AVP. Medications such as demeclocycline, lithium, amphotericin B, and fluoride [5], and electrolyte abnormalities such as hypokalemia and hypercalcemia [6] are known to cause or precipitate nephrogenic DI. Central DI occurs due to destruction of the posterior pituitary and eventually lack of AVP production or release. Without treatment, intravascular volume depletion occurs, cardiac stroke volume decreases, and eventually heart rate increases. These patients will have orthostatic hypotension, weak pulses, rapid breathing, and decreased level of consciousness. They may present with seizures if significant hypernatremia is present.
A child undergoing procedural sedation should receive his usual morning dose of desmopressin. The sedation provider should pay attention to fluid management in the patient on desmopressin therapy, as some degree of fluid restriction is required. Intravenous fluids (use 5 % dextrose-0.9 % saline) should total 1 L/m2/24 h to approximate insensible losses and obligate urine output. Oral fluids may be offered once the child is awake.
Mitochondrial Disease
Mitochondrial disease (MD) is a group of disorders that arise from defects in the oxidative phosphorylation or electron transport chain involved in generation of ATP [65]. Primary mitochondrial disorder is caused by deletions in nuclear DNA or mitochondrial DNA. Secondary disorders are due to mitochondria dysfunction caused by various drugs and by free radicals.
The ten most common syndromes associated with MD are: Kearns-Sayre syndrome; Leigh syndrome; mitochondrial DNA depletion syndrome; mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes (MELAS); myoclonic epilepsy with ragged red fibers; neurogastrointestinal encephalomyopathy, neuropathy, ataxia and retinitis pigmentosa (NARP); and external ophthalmoplegia. There is no definitive treatment for MD, although some patients improve with specific therapies such as coenzyme Q10; those with seizures may respond to a ketogenic diet.
MD may present with any symptom in any organ at any age, but some symptoms and signs are more suggestive of a mitochondrial disorder than others. These red-flag features require the initiation of a diagnostic evaluation for mitochondrial disease (Table 4.6).
Table 4.6
Factors that warrant initiation of a diagnostic evaluation in mitochondrial disease
Possible indicators of mitochondrial disease |
|---|
Neurologic |
• Nonvascular pattern for cerebral stroke-like lesions |
• Basal ganglia diseases |
• Encephalopathy—either recurrent or induced by low or moderate dosing of valproate |
• Neurodegeneration |
• Epilepsia partialis continua (Kojevnikov’s epilepsia) |
• Myoclonus |
• Ataxia |
• Magnetic resonance imaging consistent with Leigh disease |
• Characteristic magnetic resonance spectroscopy peaks: |
– Lactate peak at 1.3 ppm TE (echo time) at 35 and 135 ms |
– Succinate peak at 2.4 ppm |
Cardiovascular |
• Hypertrophic cardiomyopathy with rhythm disturbance |
• In a child: unexplained heart block |
• Cardiomyopathy combined with lactic acidosis (>5 mM) |
• Dilated cardiomyopathy combined with muscle weakness |
• Wolff-Parkinson-White arrhythmia |
Ophthalmologic |
• Retinal degeneration. May include: |
– Decreased visual acuity |
– Night blindness |
– Deficits in color vision |
– Pigmentary retinopathy |
• Ophthalmoplegia/paresis |
• Disconjugate movement of eyes |
• Ptosis |
• Sudden-onset or insidious-onset optic neuropathy or atrophy |
Gastroenterologic |
• Liver failure: unexplained or valproate-induced |
• Severe dysmotility |
• Pseudo-obstructive episodes |
Other red flags |
• Newborn, infant, or young child experiencing: |
– Unexplained hypotonia |
– Weakness |
– Failure to thrive |
– Metabolic acidosis (particularly lactic acidosis) |
• Exercise intolerance disproportionate to weakness |
• Hypersensitivity to general anesthesia |
• Acute rhabdomyolysis |
Sedating-anesthetizing children with MD may perplex many practitioners. Currently there is no clear evidence-based guidance in the literature regarding the anesthetic-sedative management of these patients. Complicating matters further is the risk of clinical deterioration related to the stress of the procedure itself, unrelated to nature of the anesthetic-sedative agents used. It is well known that children with mitochondrial defects (MD) may have an increased risk for cardiorespiratory and neurological and metabolic complications from anesthesia-sedation. Any organ may be affected in MD: meticulous individualized pre-sedation assessment is essential. Sedation providers should review and consider obtaining complete blood count, basic metabolic panel, liver function tests, thyroid function tests, sleep studies, and ECG and/or echocardiogram as indicated by the patient’s condition and the associated syndrome.
Patients with MD often develop hypoglycemia and lactic acidosis, which can be exacerbated by the stress of the procedure. Hypoglycemia is common: diseased mitochondria cannot keep up with the body’s energy requirements via fatty acid oxidation during stress, which leads to drawing on and rapid depletion of carbohydrate stores. Minimizing periods of fasting and routine use of lactate-free intravenous fluids (such as 5 % dextrose-0.9 % saline) in all patients with MD undergoing sedation-anesthesia is recommended. Prolonged procedure time requires lactate and blood glucose monitoring. This is especially important for infants, as glucose is the major energy supply to the myocardium, and hypoglycemia may contribute to myocardial depression.
The prevalence of cardiomyopathy in children with MD is reported to be 20 % [66, 67]. The severity of MD correlates with the severity of impairment of cardiac function. Cardiac impairment occurs in Barth syndrome, Kearns-Sayre syndrome, ocular myopathy, and MELAS. A pre-procedure baseline ECG is strongly recommended and can be extremely valuable; red flags in the ECG include any form of heart block or prolonged QT. If the screening ECG is abnormal, a cardiology consult is recommended before proceeding with elective sedation-anesthesia in these patients. For those with cardiomyopathy, an echocardiogram within the past year is recommended.
There is no absolute contraindication to any particular anesthetic-sedative agent for patients with MD. Many anesthetic agents adversely affect mitochondrial function in vitro but adverse events in vivo are only sparsely reported. Furthermore, the anesthetic agents implicated in these cases have been used without incident in many other reports. Opioids, ketamine, midazolam, and dexmedetomidine do not appear to inhibit mitochondrial function. At the present time there is no need to avoid volatile agents in patients with MD; inhalational anesthetics have been used without ill effects in these children. Keep in mind that patients with MD may have impaired upper airway and respiratory response to hypoxia and hypercarbia. Sedative agents should be titrated carefully in order to avoid respiratory depression.
Patients with MD may be more susceptible to the effects of lipophilic agents such as propofol. Propofol uncouples oxidative phosphorylation in mitochondria and suppresses ATP production by interfering with the electron transport chain [68]. There are cases in which short-term use of propofol has resulted in propofol infusion syndrome (acute bradycardia resistant to treatment and progressing to asystole). These patients may have subclinical forms of mitochondrial disease that are uncovered by the infusion of propofol. Single dose propofol has been used safely in many patients, but the true risk associated with this practice and the safe total dose and duration of infusion is not established. Since there are many sedative-anesthetic alternatives, it is reasonable to avoid the use of propofol infusion in these patients.
As in any child with a known myopathy, children with MD are at risk at baseline for rhabdomyolysis. Further, due to abnormal neuromuscular endplates with the subsequent risk of hyperkalemia, a depolarizing agent such as succinylcholine is contraindicated. Note also that patients with MD also exhibit variable sensitivity to the non-depolarizing neuromuscular blocking agents. Many report mitochondrial patients’ experiencing prolonged neuromuscular block with non-depolarizing neuromuscular blocking agents. Careful titration of neuromuscular blocking agents by twitch monitoring and consideration of administration of reversal agents are recommended.
To summarize, the most important anesthetic-sedative considerations in these patient are: to maintain normoglycemia and normothermia, to avoid any period of hypoxia, to maintain normovolemia, and to avoid metabolic stresses that can lead to or worsen lactic acidosis.
Multiple Allergies
The term “drug allergy” is often misused by clinicians and patients to describe any reaction (proven or perceived) to a medication. The preferred general term is adverse drug reaction, which encompasses the important subcategories. Three clinically relevant subcategories are: drug allergy (reaction resulting from an immunologic mechanism), drug intolerance (reaction resulting from non-immunologic and/or unknown reasons), and pseudo-allergy (reaction resembling allergy, but with a multifactorial, unknown, or idiosyncratic cause) [69].
It may not be feasible to differentiate the above in the pre-sedation assessment [70]. Allergists suggest referring to these events as predictable reactions (drug overdose, side effects, drug–drug interactions) and unpredictable reactions (allergy, intolerance, pseudo allergy). Predictable reactions are often benign, and account for approximately 80 % of adverse drug reactions. Unpredictable reactions account for the remaining 20 %, with allergic or pseudo-allergic reactions comprising 5–10 % of adverse drug reactions [69].
Confirming the diagnosis of a drug allergy is not the goal of the pre-sedation assessment; drug provocation testing performed in other settings remains the criterion standard. However, it is important to note that drug allergy is over-diagnosed in children [71]. Although it is prudent to avoid drugs that may have provoked some reaction in the past, when few alternatives remain the clinician should focus on determining the risk and potential severity of unpredictable reactions during sedation. Type I allergic reactions are immediate and due to drug-specific antibodies; they require prior exposure and sensitization to the drug. Clinical manifestations include urticaria, angioedema, bronchospasm, and/or anaphylaxis. Type II reactions (anti-tissue cytotoxic, e.g., hemolytic anemia or thrombocytopenia) and Type III reactions (immune complex, e.g., serum sickness) are readily identified by a history of severe illness or hospitalization. Type IV reactions (the most common) are delayed hypersensitivity reactions evolving over hours to days, and often present with maculopapular exanthems (but may also manifest as eczematous, pustular, or bullous lesions) [69].
Documenting the timing, course of the reaction, and likely inciting drug may help the clinician to understand the safety of the use of the proposed medication during the procedure. Electronic medical records may be a good source of information, as many include entries on when the drug was given and the nature of the reaction [72].
Multiple drug allergy syndrome (MDAS) describes a condition in which the patient experiences allergic or pseudo-allergic reactions to related and non-related drugs [73]. Most cases involve urticarial and/or angioedema; however, Stevens-Johnson syndrome and anaphylaxis have been reported. Interestingly, skin testing in these patients may be negative, even after significant clinical manifestations have been documented. These patients typically are older, most are adults, and many have multiple comorbidities and a long past medical history (with many opportunities to become sensitized to many different types of drugs). Information about the pathophysiology of MDAS remains limited, as there is no criterion standard for diagnosis and prospective studies are lacking [70].
Multiple drug intolerance syndrome (MDIS) may be a separate entity from that which is described above. MDIS is defined as a hypersensitivity to three or more drugs that are “chemically, pharmacologically, and immunogenically unrelated, taken on three different occasions, and with negative allergy skin tests” [74, 75]. MDIS patients are also typically older, have anxiety, depressive and/or somatoform symptoms, and are typically convinced that they are allergic to all drugs. These patients often require allergy and psychiatric consultations as an outpatient [76].
In summary, the pre-sedation assessment should focus on true allergic or pseudo-allergic signs or symptoms associated with a particular drug and the severity of the presentation. When in doubt and feasible, the clinician in this setting may avoid the drug altogether. If there is a conflict or no acceptable alternative, a frank discussion about the risks, benefits, and other possible alternatives is needed.
Muscular Dystrophies
The muscular dystrophies (MD) are a group of progressive myopathic disorders characterized by muscle wasting and weakness. The most common are Duchenne and Becker MDs; other types present at different stages in life, with varying degrees of severity and involving different muscle groups: fascioscapulohumeral, limb-girdle, distal, oculopharyngeal, and Emery-Dreifuss [77]. The morbidity of the most common, Duchenne and Becker MDs, involves progressive respiratory failure with recurrent lung infections.
The disease is characterized by severe proximal muscle weakness, progressive degeneration, and fatty infiltration of the muscles. Symptoms typically appear at the age of 2–6 years; delayed walking beyond 15 months of age is a common initial sign. Affected children never run properly and have difficulty climbing stairs; only approximately 10 % manage to jump with both feet together. Many children require the use of a wheelchair by age 12, and may not live past their 20s [77]. Most MDs involve some degree of cardiomyopathy and all are at risk for heart failure [78]. Other manifestations include pseudohypertrophy of the calves and markedly elevated creatine kinase levels. The progressive nature of the disorder results in restrictive pulmonary disease, multiple contractures, and scoliosis. Due to advances in medical management, many of these patients may now be expected to live into adulthood.
The pre-procedure assessment should focus on the child’s overall function (ambulatory or wheelchair) with careful attention to respiratory toilet. The child with disturbed sleep, nightmares, daytime drowsiness, or early morning headaches may have unrecognized nocturnal hypoventilation. This may be a clue to a recent worsening trajectory of illness and make the child more likely to benefit from noninvasive positive pressure ventilation during sleep or sedation. Worsening respiratory symptoms may preclude outpatient sedation.
Symptoms of dizziness, chest pain, intermittent increased shortness of breath, nausea, and decreased oral intake may be consistent with developing (or worsening) cardiomyopathy. A thorough cardiovascular exam with careful attention to signs of heart failure (hepatic congestion in infants and toddlers, facial and extremity edema in older children; presence of an S3 or precordial heave) is warranted. One-third of these patients have dilated cardiomyopathy by age 14, with nearly all patients developing some degree of cardiomyopathy by age 18. Due to the prevalence of cardiac disorders in these patients, the American Academy of Pediatrics recommends that children with DMD should undergo cardiac evaluation and optimization of cardiovascular status prior to elective anesthesia [79].
While it is important to investigate and optimize cardiovascular status before the elective procedure, these patients can develop complications despite the presence of reassuring pre-procedure tests. Unexplained tachycardia should raise the suspicion of cardiomyopathy. A pre-procedure baseline ECG and potentially an echocardiographic assessment (within a year from the date of the procedure) are recommended to optimize cardiac function and avoid a dysrhythmia. A child with a pre-procedure echocardiogram showing good left ventricular function may not respond adequately to myocardial stress during the procedure. Some children with particular MDs are at higher risk for dysrhythmias, and require a prophylactic implantable defibrillator [80]. The severity and progression of skeletal muscular disease may be outpaced by worsening cardiac muscular disease, such as non-ischemic cardiomyopathy [81].
Another important concern in these patients is careful evaluation of the airway and respiratory apparatus. These patients may have a difficult airway due to a combination of macroglossia, weak upper respiratory muscles, limited cervical spine mobility, and limited mandibular mobility. DMD is characterized by weakness of the diaphragm, intercostal muscles, and the accessory muscles of respiration, resulting in restrictive pulmonary impairment and a progressive decrease in total lung capacity and vital capacity. For patients with declining respiratory function, it may be necessary to prepare for noninvasive ventilation prior to the procedure.
During sedation, patients with MD are at risk for rhabdomyolysis, with subsequent acute renal failure or hyperkalemia. A careful review of the child’s past procedures and outcomes is recommended. Ideally the child is euvolemic prior to the procedure; care should be taken for proper positioning and potentially adjusting positions during long procedures to discourage the development of rhabdomyolysis. Keep in mind that children with MDs are often sensitive to small doses of opioids and sedatives, which may cause a sudden and prolonged apnea [82]. Plan for minimum pre-sedation and small titratable aliquots.
Controversy exists concerning the role of inhalational anesthetics and succinylcholine in “triggering” rhabdomyolysis or malignant hyperthermia [78, 83–85]. Some experts recommend against their use based on case reports. Many clinicians avoid their use altogether in children with MD. Propofol, dexmedetomidine, and ketamine (among others) have all been used with success in intravenous sedation in these children [78, 86–88]. Nitrous oxide may be considered in children with MD without significant cardiomyopathy or cardiac dysfunction [66].
Musculoskeletal Disorders
Children with musculoskeletal disorders may present repeatedly for diagnostic procedures. These children should be managed with sensitivity. Positioning for the procedure can be challenging, especially in those with limb deformities and contractures. Whenever possible, offer the child a position of comfort and minimize focal pressure during sedation.
Achondroplasia is the most common nonlethal skeletal dysplasia. There are two causes for this disorder: the child has either a de novo mutation of the fibroblast growth factor receptor 3 gene or inherits the disorder from his parents. These patients have midface hypoplasia, a depressed nasal base, small nasal airways, narrow oropharynx, and upper airway muscle hypotonia, which predispose them to development of obstructive sleep apnea (OSA) [89]. They tend to have a large head, a bell-shaped chest, cupping of the ribs, and short arms and legs.
Sedative-anesthetic risks in these patients include a challenging airway and increased sensitivity to sedative-anesthetic agents. Patients with severe kyphoscoliosis and restrictive lung disease may have baseline hypoxemia and low lung volumes, predisposing them to hypoxemia during sedation. Review of CT scans and MRI of the spine is helpful before sedating these children. Hyperextension of the neck should be avoided and special consideration should be taken before manipulating the neck due to the possibility of cervical cord compression [90].
The sedation practitioner must be aware of potential complications when sedating a patient with history of significant scoliosis. The primary aim of pre-procedure evaluation is to detect the presence and extent of cardiac or pulmonary compromise. The earlier the age of onset and the more immature the bone growth at the time the process begins, the worse the disease burden. Children with idiopathic scoliosis tend to have less pulmonary embarrassment than children with neuromuscular scoliosis, who may have abnormalities in the central control of breathing and impaired airway reflexes. Poor coordination of laryngeal and pharyngeal muscles may result in abnormal control of secretions and inadequate cough, increasing the risk of aspiration.
Respiratory function should be assessed by a thorough history, focusing on functional impairment (exercise tolerance). Physical examination should include a good understanding of vital capacity (review any pulmonary function tests that may be available). If pre-procedure vital capacity is less than 30–35 % of predicted, post-procedure ventilation is likely to be required. Cardiac dysfunction may occur in scoliosis from distortion of the mediastinum; patients may develop cor pulmonale from chronic hypoxemia and pulmonary hypertension. Cardiac studies (ECG, echocardiogram) may be performed as indicated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


