Chapter 4
The Placenta
Anatomy, Physiology, and Transfer of Drugs
Mark I. Zakowski MD, Andrew Geller MD
Chapter Outline
The placenta is a critical organ of great importance to obstetric anesthesia. Revered by ancient cultures as “the seat of the external soul” or “the bundle of life,” the placenta is involved in many cultural rituals.1 However, understanding of the indispensable role of the placenta in the development of the fetus did not start to evolve until the 17th century and continues today via microanatomic, biochemical, and molecular biologic techniques. The concept of the placenta as a passive sieve (acting only as a conduit for oxygen, nutrients, and waste) has been dispelled with the realization that the placenta is a complex and dynamic organ. Indeed, new studies show the critical importance of placental function in the metabolism, nutrition, and hormonal maintenance of pregnancy. Maternal-placental conditions can affect the fetus not only during pregnancy but also in adulthood and beyond into the next generation via epigenetic mechanisms.2
The placenta brings the maternal and fetal circulations into close apposition without substantial interchange of maternal and fetal blood for the physiologic transfer of gases, nutrients, and wastes. This important exchange is accomplished within a complex structure that is almost entirely of fetal origin.
Anatomy
Embryology
The blastocyst initially attaches to endometrial pinopodes (uterodomes), which express markers of endometrial receptivity (e.g., galectin-9).3 The remodeling of uterine extracellular matrix starts with serine proteases and metalloproteinases (e.g., MMP-2 and MMP-9). The developing blastocyst erodes the surrounding decidua, leaving the cellular debris on which it survives. The syncytiotrophoblasts (invasive cells located at the margin of the growing conceptus) continue to erode the surrounding decidua and its associated capillaries and arterioles until the blastocyst is surrounded by a sea of circulating maternal blood (trophoblastic lacunae). The vitelline vein system develops in the yolk sac of the embryo to enhance the transport of nutrients, which diffuse from the maternal blood through the trophoblast layer and chorionic plate into the chorionic cavity. The embryo undergoes a dramatic acceleration in growth as its dependence on simple diffusion diminishes.4
At 2 weeks of development, the primitive extraembryonic mesoderm (cytotrophoblast layer) begins to proliferate as cellular columns into the syncytiotrophoblast. These columns with their syncytiotrophoblast covering extend into the maternal blood lacunae and represent primary villi. Further mesodermal invasion into the core of these primary villi marks the metamorphosis into secondary villi. Cellular differentiation of the villi mesoderm results in the formation of a network of blood cells and vessels; this transition allows their classification as tertiary villi. The vascular components of each villus develop connections within the chorionic plate and into the stalk that connects the developing embryo and primitive placenta. Penetration of the cytotrophoblast continues through the syncytiotrophoblastic layer until many of the villi reach the decidua and form anchoring villi (Figure 4-1).4,5
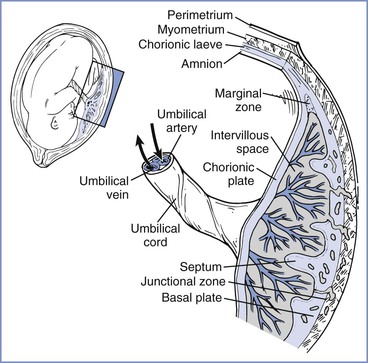
FIGURE 4-1 The placenta is a complex structure that brings the maternal and fetal circulations into close apposition for exchange of substances. (Redrawn from Kaufmann P, Hans-Georg F. Placental development. In Polin RA, Fox WW, Abman SH, editors. Fetal and Neonatal Physiology. 3rd edition. Philadelphia, Saunders, 2004:85-96.)
Villi continue to develop and undergo extensive branching into treelike structures; the branches, which extend into the lacunar (or intervillous) spaces, enlarge the surface area available for exchange. Further villous maturation results in a marked reduction in the cytotrophoblastic component and a shortening of the distance between the fetal villi and maternal intervillous blood.4
The growing embryo within the blastocyst attaches to the chorion through a connecting or body stalk. Mesodermal components of this stalk coalesce to form the allantoic (or rudimentary umbilical) vessels. As the embryo continues its exponential growth phase, the connecting stalk shifts ventrally from its initial posterior attachment. The expansive open region at the ventral surface of the embryo constricts as the body wall grows and closes. By so doing, the body wall surrounds the yolk stalk, allantois, and developing vessels within the connecting stalk to form the primitive umbilicus. As the expanding amnion surrounds and applies itself over the connecting stalk and yolk sac, the cylindrical umbilical cord takes on its mature form.4
Placental development is a dynamic process influenced by many factors. Nitric oxide plays an important role in embryo development, implantation, and trophoblast invasion in diverse species.6 Human endothelial nitric oxide synthase (eNOS) expression in the syncytiotrophoblast and early endothelium occurs in the first trimester. Later in pregnancy, eNOS increases and becomes more prominent in the syncytiotrophoblast and endothelial cells. Vasculogenesis and angiogenesis depend on vascular endothelial growth factor (VEGF) and its receptors VEGFR-1 (Flt-1) and VEGFR-2 (Flk-2), transforming growth factor-β1 (TGF-β1), and angiopoietin 1 and 2, which exert their effects in part through nitric oxide. Hypoxia also plays an important role in placental development and angiogenesis by stimulating trophoblast invasion and differentiation via hypoxia-inducible factor-alpha, which activates VEGF and eNOS. Relative hypoxia must be maintained in early placental development because the placental-fetal unit cannot tolerate the oxidative stress of reactive oxygen species during organogenesis.7 Oxygen levels influence the placental vascular sensitivity to vasodilators and constrictors. In vitro studies have shown that NOS inhibition and hypoxia independently increase placental perfusion pressure. Both of these effects are prevented by nitric oxide donors, suggesting a common pathway with the effect of hypoxia mediated partly by low NOS activity.6
The development of preeclampsia is related, at least in part, to abnormal placental growth and implantation at this early stage of development (see Chapter 36). In patients with preeclampsia, the villous tree has longer capillaries with fewer branches.6 Vascular dysfunction occurs mainly from changes in vascular structure and activation of nitric oxide synthesis rather than from altered responses to nitric oxide and vasoconstrictors.
DNA, gene expression, and manipulation of gene expression control placental development, fetal development, adult phenotype expression, and clinical diseases, even into subsequent generations.2,8 The evolving field of epigenetics explores the prolonged effect of maternal and paternal environmental influences; gene expression becomes altered by DNA methylation, histone modification, and noncoding RNA. At fertilization, global DNA methylation is erased so at the blastocyst stage (implantation) the genome is hypomethylated.8 DNA methylation occurs in a specific manner so the trophectoderm (which becomes the placenta) remains relatively hypomethylated (50% to 70%) compared with the inner cell mass tissue (which becomes somatic human tissue). Genomic imprinting causes the silencing of one allele-specific copy of a gene. DNA methylation of imprinted genes occurs at the germ cell stage but is not involved in the methylation remodeling. Indeed, the human placenta exhibits extensive intraplacental mosaicism in an X-chromosome inactivation pattern. Individual placental cotyledons are derived from only a few cells, leading to cotyledon mosaicism. Even the process of in vitro fertilization produces an altered imprinted gene methylation pattern in the placenta.
Altered gene methylation has been linked to clinical disease states.8 Increased long interspersed nuclear element-1 (LINE1) gene methylation is associated with early-onset preeclampsia. Compared with disease-free matched tissue, early-onset preeclampsia is associated with hypomethylation of 34 specific genes, whereas only 4 hypomethylated genes were associated with late-onset preeclampsia.9 Thus, DNA and DNA regulatory changes influence not only early placental development but also the occurrence of pregnancy-associated disease.
Human studies have demonstrated fetal programming of childhood and adult disease. For example, a study showed that adults who were exposed in utero to episodes of malnutrition developed reduced glucose tolerance, atherogenic lipid profiles, and a doubled rate of cardiovascular diseases; these disease states were associated with hypomethylation of regulatory areas for insulin-like growth factor-2 and other genes.2 In utero exposure to a high-fat diet can lead to an increased incidence of diabetes in offspring. Maternal stress during pregnancy can lead to infant neurodevelopmental disorders.2
The placenta grows dramatically from the third month of gestation until term, with a direct correlation between placental growth and fetal growth. By term, the mature placenta is oval and flat, with an average diameter of 18.5 cm, weight of 500 g, and thickness of 23 mm. At term, the human fetal-placental weight constitutes 6% of maternal weight. Placental weight increases 0.7% per day, with active fetal growth contributing up to 1.5% of fetal body mass per day.10 The allocation of nutrient and metabolic resources for fetal growth potentially come at the expense of the mother. The growth of the placenta and fetus is influenced by maternal anabolic status, placental growth hormone, insulin-like growth factor-1, leptin, and glucocorticoids.11 Whether maternal or fetal in origin, increased glucocorticoids signal adverse environmental conditions and result in reduced glucose and amino acid transfer to the fetus. Indeed, competition between mother and fetus for resource allocation has been termed the kinship theory, in which imprinted genes influence the balance of nutrient allocation in a context-specific manner.11
Comparative Anatomy
The placentas of different species differ greatly, beginning with their method of uterine attachment, which can include adhesion, interdigitation, and fusion. In addition, the number of tissue layers between the maternal and fetal circulations differ. The most commonly used placental categorization system, the Grossner classification, uses the number of tissue layers in the placental barrier to help differentiate species (Figure 4-2).12
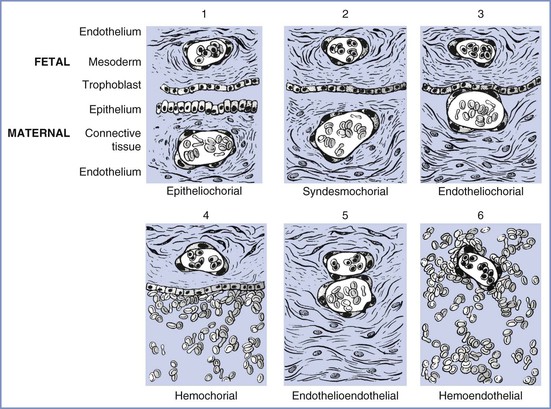
FIGURE 4-2 Modification of Grossner’s original classification scheme, showing the number and types of tissue layers between the fetal and maternal circulations. Examples of each are as follows: (1) epitheliochorial, sheep; (2) syndesmochorial, no known examples; (3) endotheliochorial, dogs and cats; (4) hemochorial, human and hamster; (5) endothelioendothelial, bandicoot (Australian opossum); and (6) hemoendothelial, Rocky Mountain pika. (Modified from Ramsey EM. The Placenta: Human and Animal. New York, Praeger Publishers, 1982.)
The ability of the placenta to transfer various substances differs among species. The markedly thicker epitheliochorial placenta found in sheep, a species commonly used for placental transfer studies, has three maternal layers (epithelium, connective tissue, and endothelium) that separate maternal from fetal blood. By contrast, the human hemochorial placenta lacks these maternal layers, which allows maternal blood to bathe fetal tissues directly (see Figure 4-2). As a result, species differ in the transfer of substances through the placenta; for example, fatty acids cannot cross through the placenta in sheep as they do in humans.13 This wide diversity in placental structure and function among species makes extrapolation from animal investigations to clinical medicine tenuous.
Vascular Architecture
Maternal
Under the initial hormonal influences of the corpus luteum, the spiral arteries of the uterus become elongated and more extensively coiled. In the area beneath the developing conceptus, the compression and erosion of the decidua induces lateral looping of the already convoluted spiral arteries,14 accessing the intervillous spaces. In late pregnancy, the growing demands of the developing fetus use approximately 200 spiral arteries that directly feed the placenta to handle a blood flow of approximately 600 mL/min.14 The vasodilation required to accommodate this flow is the result of the replacement of the elastic and muscle components of the artery, initially by cytotrophoblast cells and later by fibroid cells. This replacement reduces the vasoconstrictor activity of these arteries and exposes the vessels to the dilating forces of the greater blood volume of pregnancy, especially at the terminal segments, where they form funnel-shaped sacs that enter the intervillous space.14 The increased diameter of the vessels decreases blood velocity and reduces blood pressure.
The intervillous space is a large cavernous expanse that develops from the fusion of the trophoblastic lacunae and the erosion of the decidua by the expanding blastocyst, forming a huge blood sinus bounded by the chorionic plate and the decidua basalis (i.e., the maternal or basal plate). Folds in the basal plate form septa that separate the space into 13 to 30 anatomic compartments known as lobules. Each lobule contains numerous villous trees that are also known as cotyledons or placentomes. Although tightly packed with highly branched villous trees, the intervillous space of the mature placenta can accommodate approximately 350 mL of maternal blood.
Maternal arterial blood leaves the funnel-shaped spiral arteries and enters the intervillous space. The blood moves into the nearly hollow, low-resistance area, where villi are very loosely packed (the intercotyledonary space), before entering another region of densely packed intermittent and terminal villi (Figure 4-3).15 The terminal villi represent the areas where placental exchange predominates. After passing through this dense region, maternal venous blood collects between neighboring villous trees in an area called the perilobular zone.16 Collecting veins penetrate the maternal plate at the periphery of the villous trees to drain perilobular blood from the intervillous space.
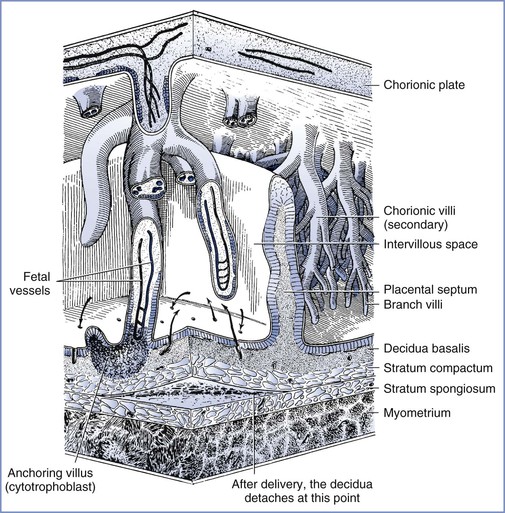
FIGURE 4-3 The relationship between the villous tree and maternal blood flow. Arrows indicate the maternal blood flow from the spiral arteries into the intervillous space and out through the spiral veins. (Modified from Tuchmann-Duplessis H, David G, Haegel P. Illustrated Human Embryology. Volume 1. Embryogenesis. New York, Springer Verlag, 1972:73.)
Fetal
Two coiled arteries bring fetal blood within the umbilical cord toward the placenta. On the placental surface, these arteries divide into chorionic arteries that supply the 50 villous trees located in the placental lobules. At the base of each villous tree, the chorionic arteries are considered the main villous stem or truncal arteries (first-order vessels), which in turn branch into four to eight ramal or cotyledonary arteries (second-order vessels); as they pass toward the maternal plate, they further subdivide into ramulus chorii (third-order vessels) and, finally, terminal arterioles. The terminal arterioles lead through a neck region into a bulbous enlargement where they form two to four narrow capillary loops. Here the large endothelial surface area and the near-absence of connective tissue allow optimal maternal-fetal exchange (Figure 4-4).16,17
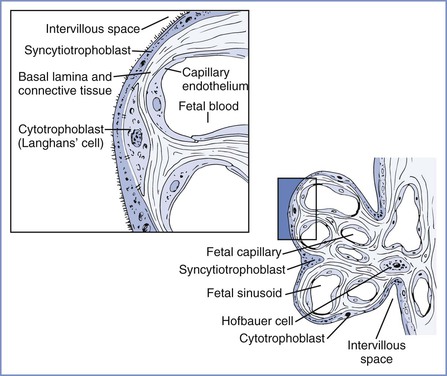
FIGURE 4-4 Left, Cellular morphology of two terminal villi. Right, Higher magnification of the boxed region exhibiting the placental barrier between fetal and maternal blood. (Redrawn from Kaufmann P. Basic morphology of the fetal and maternal circuits in the human placenta. Contrib Gynecol Obstet 1985; 13:5-17.)
The venous end of the capillaries loops, narrows, and returns through the neck region to the collecting venules, which coalesce to form the larger veins in the stem of the villous trees. Each villous tree drains into a large vein, which, as it perforates the chorionic plate, becomes a chorionic vein. All of the venous tributaries course toward the umbilical cord attachment site, where they empty into the one umbilical vein that delivers blood back to the fetus.
Physiology
Barrier Function
The placenta is an imperfect barrier that allows many substances to cross from the maternal to the fetal circulation and from the fetal to the maternal circulation. The rate and amount of placental transfer depend on the permeability and the ability of various mechanisms to restrict movement. A vast array of cytochrome P450 isoenzymes and transporters are found within the placenta; some of these are inducible, whereas others are constitutive. In addition, a number of substances undergo specific or nonspecific binding within the placental tissues, thereby minimizing fetal exposure to and accumulation of the substances. Finally, the thickness of the placental membranes, which diminishes as gestation progresses, may influence the rate of diffusion.18 Of interest, the rate of transfer of certain substances (e.g., glucose, water) differs very little among species, even though the placental thickness varies greatly.19
Fetal cells have been detected in maternal circulation, before organogenesis and full maternal arterial perfusion of the placenta, and maternal cells have also been shown to enter the fetal circulation.20 Maternal-fetal cell transfer may occur by disruption of the trophoblastic layer or by active adhesion and transmigration (similar mechanism to blood-brain barrier migration). Fetal cells may be pluripotent, and the DNA may be found in maternal organs for decades. Murine fetal progenitor cells have been found to migrate and assist with maternal wound healing.21 These microchimeric fetal cells may contribute to maternal immunomodulation, development or worsening of autoimmune diseases (e.g., thyroiditis, lupus, and asthma), and healing of wounds, including neuronal tissue.22 Indeed, placental exosomes, nanovesicles 30 to 100 nm in size found in maternal circulation that contain proteins and transcription-related materials, exert a maternal immunosuppressive effect. Placental microparticles, vesicular products of syncytiotrophoblast greater than 100 nm, also contain RNA and DNA fragments and affect fetal and maternal apoptosis, angiogenesis, and inflammation. An excess of microparticles has been observed in early-onset preeclampsia. The placenta and fetal-maternal interactions are certainly complex and worthy of further study.
Cell-free fetal DNA has been shown to be present in the plasma of pregnant women.23 This discovery has facilitated the development of a range of noninvasive diagnostic investigations, including tests for fetal sex assessment, fetal rhesus D blood group genotyping, fetal chromosomal aneuploidy detection, and other genetic abnormalities.24
Hormonal Function
A sophisticated transfer of precursor and intermediate compounds in the maternal-fetal-placental unit allows placental enzymes to convert steroid precursors into estrogen and progesterone. This steroidogenic function of the placenta begins very early in pregnancy; by 35 to 47 days after ovulation, the placental production of estrogen and progesterone exceeds that of the corpus luteum (i.e., the ovarian-placental shift).25
The placenta also produces a wide array of enzymes, binding proteins, and polypeptide hormones. For example, the placenta produces human chorionic gonadotropin, human placental lactogen (a growth hormone also known as human chorionic somatomammotropin), and factors that control hypothalamic function.25 This ability to produce proteins and steroid hormones allows the placenta to influence and control the fetal environment.26
Regulation of Placental Blood Flow
Maternal Blood Flow
The trophoblastic invasion and functional denervation of the musculoelastic lining of the spiral arteries may represent adaptive mechanisms to decrease vascular reactivity and promote vasodilation. These alterations allow the spiral arteries to vasodilate as much as 10 times their normal diameter, thereby lowering resistance for the passage of blood through the intervillous spaces.27
Maternal blood enters the intervillous cotyledon space at a pressure of 70 to 80 mm Hg in an area that has relatively few villi.14 The pressure and velocity of blood flow rapidly diminishes to approximately 10 mm Hg as the blood passes into an area of higher resistance created by the densely packed villi of the placentome.18
Fetal Blood Flow
In contrast to maternoplacental blood flow, the gestational increases in fetoplacental blood flow primarily results from vascular growth rather than vasodilation of the villous beds. Fetal perfusion of the placenta is not classically autoregulated; the placental vasculature has no innervation by the sympathetic nervous system. However, the fetus can modulate fetoplacental perfusion in a number of ways: (1) via endocrine effects of adrenomedullin, (2) via net efflux/influx of water regulated by fetal blood pressure, and (3) via local autoregulatory effects mediated by the paracrine vasodilators nitric oxide and acetylcholine.28,29 Adrenomedullin release by the fetal adrenal glands assists in maintenance of tone in placental vessels. Fetal blood pressure changes cause net influx/efflux of water across the placenta that affects fetal intravascular volume and perfusion. Maternal hyperglycemia30 and hypoxemia31 are examples of derangements that can alter regional fetal blood flow, probably through vascular mediators. Endothelium-derived relaxing factors, especially prostacyclin32 and nitric oxide,33 are important in the control of fetoplacental circulation. Hypoxia-induced fetoplacental vasoconstriction is mediated by a reduction in the basal release of nitric oxide.34 This vasoconstrictor activity is functionally similar to that found in the lung (i.e., hypoxic pulmonary vasoconstriction) and allows optimal fetal oxygenation through redistribution of fetal blood flow to better-perfused lobules.31 The placental vasculature constricts in response to graded hypoxia.35
Transport Mechanisms
Substances are transferred across the placenta by one of several mechanisms.
Passive Transport
The passive transfer of molecules across a membrane depends on (1) concentration and electrochemical differences across the membrane, (2) molecular weight, (3) lipid solubility, (4) degree of ionization, and (5) membrane surface area and thickness. This process requires no expenditure of cellular energy, with transfer driven principally by the concentration gradient across a membrane. Simple transmembrane diffusion can occur either through the lipid membrane (e.g., lipophilic molecules and water) or within protein channels that traverse the lipid bilayer (e.g., charged substances such as ions) (Figure 4-5).36,37 Drugs with a molecular weight less than 600 daltons cross the placenta by passive diffusion.38
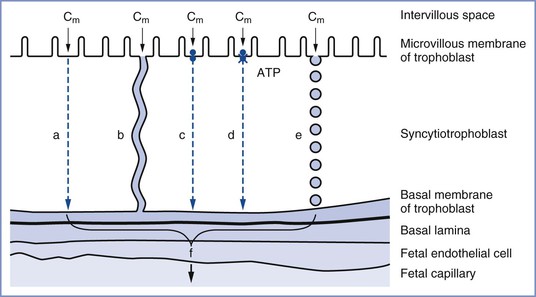
FIGURE 4-5 The transfer mechanisms used for the transfer of substances across the placental barrier: a, simple diffusion; b, simple diffusion through channels; c, facilitated diffusion; d, active transport; e, endocytosis; f, substance available for transfer into fetal circulation; Cm, intervillous concentration of substance at the trophoblastic membrane. (Modified from Atkinson DE, Boyd RDH, Sibley CP. Placental transfer. In Neill JD, Plant TM, Pfaff DW, et al., editors. Knobil and Neill’s Physiology of Reproduction. 3rd edition. St. Louis, Academic Press, 2006:2787-846.)
Facilitated Transport
Carrier-mediated adenosine triphosphate (ATP)–independent transport of relatively lipid-insoluble molecules down their concentration gradient is called facilitated diffusion.36 Facilitated diffusion differs from simple diffusion in several ways. Specifically, this mode of transfer exhibits (1) saturation kinetics, (2) competitive and noncompetitive inhibition, (3) stereospecificity, and (4) temperature influences (e.g., a higher temperature results in greater transfer). With simple diffusion, the net rate of diffusion is proportional to the difference in concentration between the two sides of the membrane. This rate limitation is valid for facilitated diffusion only when transmembrane concentration differences are small. At higher concentration gradients, a maximum rate of transfer (Vmax) is reached; thereafter, further increases in the concentration gradient do not affect the rate of transfer. The rate of transfer is determined by the number of membranous carrier protein complexes and the extent of interaction between the carrier and the substance undergoing transport.37 An example of facilitated diffusion is the transplacental transfer of glucose.
A special type of facilitated diffusion involves the “uphill” transport of a molecule linked to another substance traveling down its own concentration gradient. As such, the transfer is not directly driven by cellular energy expenditure. In most cases, sodium is the molecule that facilitates transport. For the membrane-bound carrier to transfer these molecules, both molecules must be bound to the carrier. This hybrid system is called secondary active transport or co-transport.37 The transplacental transport of amino acids appears to occur principally through secondary active transport. Transporters may be affected by disease states (e.g., preeclampsia) or signaling molecules (e.g., elevated steroid levels).39
Active Transport
Active transport involves the movement of any substance across a cell membrane; the process requires cellular energy. In general, active transport occurs against a concentration, electrical, or pressure gradient.
Like facilitated diffusion, active transport requires a protein membrane carrier that exhibits saturation kinetics and competitive inhibition.36 However, unlike secondary active transport, the movement of a substance against its concentration gradient is directly linked to the hydrolysis of high-energy phosphate bonds of ATP. The best known example of primary active transport is the translocation of sodium and potassium through the sodium-potassium adenosine triphosphatase (Na+/K+ ATPase) pump.
Active transport proteins include P-glycoprotein, breast cancer resistance protein, multidrug resistance protein, and the sodium/multivitamin transporter, as well as the many proteins involved in monoamine transport and xenobiotics.39 These transport proteins play an important role in protecting the fetus from foreign and potentially teratogenic compounds. Drugs may compete with endogenous compounds of similar shape and charge for active transport.39 P-glycoprotein transports many lipophilic drugs and antibiotics and removes cytotoxic compounds from the fetus; it exists on the maternal side of the trophoblastic cell membrane of the placenta and prevents compounds such as methadone and saquinavir (a protease inhibitor) from leaving the maternal blood, thus limiting fetal exposure.40 Inhibition of these transporter proteins (e.g., inhibition of P-glycoprotein by verapamil) can significantly increase the fetal transfer of certain drugs, including midazolam, which is a substrate for P-glycoprotein. DNA transcription of transporters may be induced by drugs or disease states. Expression of transporters may change with gestational age.41
Pinocytosis
Large macromolecules (e.g., proteins that exhibit negligible diffusion properties) can cross cell membranes via the process of pinocytosis (a type of endocytosis). Pinocytosis is an energy-requiring process in which the cell membrane invaginates around the macromolecule. Although the contents of pinocytotic vesicles are subject to intracellular digestion, electron microscopic studies have demonstrated that vesicles can move across the cytoplasm and fuse with the membrane at the opposite pole. This appears to be the mechanism by which immunoglobulin G is transferred from the maternal to the fetal circulation.36
Other Factors That Influence Placental Transport
Other factors that affect maternal-fetal exchange include (1) maternal and fetal blood flow, (2) placental binding, (3) placental metabolism, (4) diffusion capacity, (5) maternal and fetal plasma protein binding, and (6) gestational age (the placenta is more permeable in early pregnancy).42 Lipid solubility, pH gradients between the maternal and fetal environments for certain basic drugs (“ion trapping”), and alterations in maternal or fetal plasma protein concentrations found in normal pregnancy43 and other disease states (e.g., preeclampsia) may also alter placental transport.
Transfer of Respiratory Gases and Nutrients
Oxygen
Our knowledge of oxygen transfer physiology in the placenta is largely derived from the lung. The placenta must provide approximately 8 mL O2/min/kg fetal body weight for fetal growth and development, while adults only require 3 to 4 mL O2/min/kg at rest.44 As the “lung” for the fetus, the placenta has only one fifth the oxygen transfer efficiency of the adult lung.45 The human lung, with a surface area of 50 to 60 m2 and a thickness of only 0.5 µm, has a very large oxygen diffusion capacity; in comparison, the placenta has a lower diffusion capacity because of its smaller surface area of 16 m2 and a thicker membrane of 3.5 µm. Furthermore, 16% of uterine blood flow and 6% of umbilical blood flow are shunted through the placenta.18
Oxygen transfer across the placenta depends on the membrane surface area, membrane thickness, oxygen partial pressure gradient between maternal blood and fetal blood, affinity of maternal and fetal hemoglobin, and relative maternal and fetal blood flows. As physically dissolved oxygen diffuses across the villous membranes, bound oxygen is released by maternal hemoglobin in the intervillous space and diffuses across the placenta. Several factors affect the fetal blood PO2 once it reaches equilibration in the villi end-capillaries. First, the concurrent and countercurrent arrangements of maternal and fetal blood flow play a key role for placental oxygen transfer in various species. The almost complete equilibration of maternal and fetal PO2 values suggests that a concurrent (or parallel) relationship between maternal blood and fetal blood exists within the human placenta (Figure 4-6),18,46 although others have described a multivillous, cross-current flow pattern.
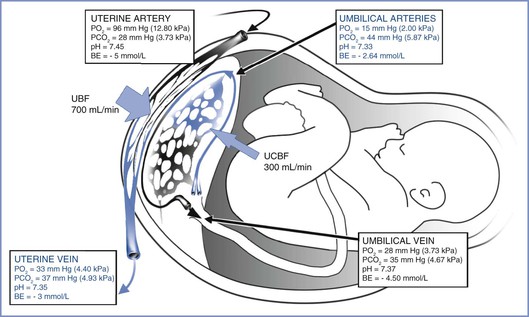
FIGURE 4-6 The concurrent relationship between the maternal and fetal circulations within the placenta and the way this arrangement affects gas transfer. These values were obtained from patients’ breathing room air during elective cesarean delivery. BE, base excess; PO2, partial pressure of oxygen; PCO2, partial pressure of carbon dioxide; UBF, uterine blood flow; UCBF, umbilical cord blood flow. (Blood gas data from Ramanathan S. Obstetric Anesthesia. Philadelphia, Lea & Febiger, 1988:27. Drawing by Naveen Nathan, MD, Northwestern University Feinberg School of Medicine, Chicago, IL.)
Much of the literature in this area is based on animal studies. Because the functional anatomy of the placenta in many mammals involves more layers than the human placenta (e.g., the epitheliochorial placenta of the sheep has three layers), results of animal models can only provide evidence for trends, not values, in humans.
In humans, oxygen solubility is 10−4 M in plasma and 10−2 M in hemoglobin; thus, 99% of the oxygen content in blood is bound to hemoglobin. With an inspired oxygen fraction of 1.0, the maximum maternal arterial PO2 was 425 mm Hg, but the fetal umbilical venous PO2 was only 47 mm Hg, indicating a low diffusion capacity of oxygen across the placenta.47 In addition, the placenta receives less than 50% of the fetal cardiac output, and blood returning from the placenta admixes with the nonoxygenated blood in the fetal inferior vena cava, thus limiting fetal arterial PO2.
Although some have called the human placenta “diffusion limited” because of the decreased ability of oxygen to cross the intervillous membrane, the delivery of oxygen to the fetus is predominantly flow limited. Maternal delivery of blood (i.e., oxygen) to the uterus is the predominant factor controlling fetal oxygen transfer. The high fetal hemoglobin concentration (17 mg/dL) accounts for the large oxygen content and the net delivery of large quantities of oxygen to the fetus. Fetal hemoglobin has a higher oxygen affinity and therefore a lower partial pressure at which it is 50% saturated (P50: 18 mm Hg) than maternal hemoglobin (P50: 27 mm Hg). This gradient produces a “sink” effect that enhances oxygen uptake by fetal red blood cells, keeping fetal PO2 lower and promoting the transfer of additional oxygen across the placenta (see Figure 5-7). The Bohr effect also augments the transfer of oxygen across the placenta. Specifically, fetal-maternal transfer of carbon dioxide makes maternal blood more acidic and fetal blood more alkalotic. These alterations of pH cause shifts in the maternal and fetal oxyhemoglobin dissociation curves, further enhancing the maternal oxygen transfer to the fetus in what is termed the “double” Bohr effect. This accounts for 2% to 8% of the transplacental transfer of oxygen.48
The placenta normally has a 50% reserve for changes in maternal or fetal blood flow by increasing venous extraction, a mechanism similar to that in adults. Based on umbilical venoarterial difference, human fetal oxygen uptake at term is 0.25 mmol/kg/min.49 The metabolic activity of the placenta itself consumes up to 40% of the oxygen uptake. Placental oxygen consumption is stable even with changes in maternal blood pressure and PO2; 30% of placental oxygen is used for protein synthesis and almost 30% for Na+/K+ ATPase. The human placenta has a villous structure, which may be an adaptation for greater maternal flow and thus oxygen delivery, but at the expense of a smaller surface area and cross-current exchange mechanism.50 However, the placenta does change in response to chronic hypoxia found at high altitudes, with an increased capillary volume and decreased capillary thickness providing a near-doubling of the oxygen diffusion capacity.51
Carbon Dioxide
The transfer of CO2 occurs through a number of different forms, including dissolved CO2, carbonic acid (H2CO3), bicarbonate ion (HCO3−), carbonate ion (CO32−), and carbaminohemoglobin. Equilibrium between CO2 and HCO3− is maintained by a reaction catalyzed by carbonic anhydrase in red blood cells. The PCO2 gradient between fetal and maternal blood (i.e., 40 versus 34 mm Hg, respectively) drives fetal-maternal transfer. Carbon dioxide is 20 times more diffusible than oxygen and readily crosses the placenta,52 although dissolved CO2 is the form that actually crosses. The rapid movement of CO2 from fetal capillary to maternal blood invokes a shift in the equilibrium of the carbonic anhydrase reaction (La Chatelier’s principle) that produces more CO2 for diffusion. The transfer of CO2 is augmented further by the production of deoxyhemoglobin in the maternal blood, which has a higher affinity for CO2 than oxyhemoglobin (the Haldane effect). The resulting affinity may account for as much as 46% of the transplacental transfer of carbon dioxide.46 Although a significant fetal-maternal concentration gradient exists for HCO3−, its charged nature impedes its transfer and contribution to CO2 transport except as a source for CO2 production through the carbonic anhydrase reaction.53
Glucose
Simple diffusion alone cannot account for the amount of glucose required to meet the demands of the placenta and fetus. To assist the movement of glucose down its concentration gradient, stereospecific-facilitated diffusion systems have been described with glucose transporters such as GLUT1 and GLUT3; the system is independent of insulin, a sodium gradient, or cellular energy.54 Insulin does not cross the placenta; however, insulin receptors in the maternal side of the syncytiotrophoblast regulate nutrient transport through a signaling cascade involving the mammalian target of rapamycin complex (mTORC). Nutrient sensors for glucose, amino acids, oxygen, cytokines, growth factors, and energy levels stimulate mTORC1, a key sensing and signaling protein in the syncytiotrophoblast that regulates nutrient transport and growth.55
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


