Fig. 2.1
Flow chart for diagnosis of LAC
Numerous variables affect screening tests for LAC detection. The low content of PL renders the test more sensitive, and KCT can be considered the most sensitive as no external PL is added to the assay, with the only available PL being those present in the plasma tested. Unfortunately, KCT is hard to automate, as most photo-optical devices cannot be used in the presence of the kaolin reagent, which tends to scatter light. The presence of platelets in this and in other assays greatly affects the results, particularly when plasma is frozen before testing. Centrifuging fresh plasma twice or using a double filter (through a 0.2-μm filter) may get around this problem. aPTT is a general coagulation screening test, KCT and dPT are used less frequently, and dRVVT has become the most commonly used screening assay for detection of LAC. We have modified the original test by diluting both the venom and the PL, thereby greatly increasing its sensitivity. Because Russell viper venom activates factor X directly, the test is normal in patients with some factor deficiency and in those with an inhibitor to factor VIII, the most common coagulation inhibitor associated with a bleeding tendency.
The performance of laboratories across the world to detect LA is a matter of concern. Misclassification of positive or negative LA plasma sample is commonly encountered in external surveys. Recently, in a survey of centralized LA diagnosis, we reported that about 33 % of plasma samples collected by thrombosis centers and labeled locally as LA positive were reported as LA negative in a central laboratory [28].
The poor laboratory performance is due to the lack of standardized tests and/or inappropriate application of the diagnostic criteria. Recently, the Brandt guidelines were updated [29]. Particular emphasis was given to several aspects discussed in this official communication. A new paragraph was dedicated to the patient selection. Testing for LAC should be limited to patients who have a significant probability of having the antiphospholipid syndrome (APS). Appropriateness to search for LAC can be graded according to clinical characteristics into low, moderate, and high.
High: unprovoked venous thromboembolism (VTE) and (unexplained) arterial thromboembolism (ATE) in young patients (<50 years of age), thrombosis at unusual sites, late pregnancy loss, any thrombosis, or pregnancy morbidity in patients with autoimmune diseases (systemic lupus erythematosus, rheumatoid arthritis, autoimmune thrombocytopenia, autoimmune hemolytic anemia)
Moderate: accidentally found prolonged aPTT in asymptomatic subjects, recurrent spontaneous early pregnancy loss, and provoked VTE in young patients
Low: VTE or ATE in elderly patients
Modalities for blood collection and processing are fully delineated, and the choice of tests is limited to dRVVT and a sensitive aPTT. Calculation of cutoff values for each diagnostic step are clearly stated. To avoid misinterpretation, it is recommended to perform laboratory procedures 1–2 weeks after discontinuation of treatment or when the INR is less than 1.5. Bridging VKA discontinuation with LMWH is recommended with the last dose of LMWH administered more than 12 h before the blood is drawn for LAC testing. LAC result should always be considered in the context of a full laboratory aPL profile comprising aCL and aβ2GPI antibodies ELISAs. The presence of medium- to high-titer aCL and aβ2GPI of the same isotype (most often IgG) is in agreement with a positive LAC and identifies patients at high risk for thrombosis and fetal losses [30]. Diagnostic steps are confirmed to be those of previous guidelines: (a) screening step, (b) mixing test, and (c) confirmatory test.
Results of screening tests are potentially suggestive of LA when their clotting times are longer than the local cutoff value. Results should be expressed as follows:
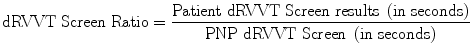 Perform testing on patient plasmas mixed with the pooled normal plasma (PNP) at 1:1 proportion and express results as follows:
Perform testing on patient plasmas mixed with the pooled normal plasma (PNP) at 1:1 proportion and express results as follows:
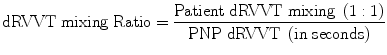 Confirmatory Test: Results are confirmatory of LA if the % correction of patient’s plasma at low (screen) and high (confirm) phospholipid concentration is above the local cutoff value.
Confirmatory Test: Results are confirmatory of LA if the % correction of patient’s plasma at low (screen) and high (confirm) phospholipid concentration is above the local cutoff value.
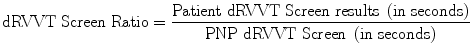
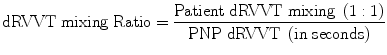
Sensitive aPTT: same procedure as dRVVT should be used.
2.5 Significance of Positive LAC
LAC is an important test in vascular medicine, since its detection on two occasions 12 weeks apart in a patient with venous or arterial thromboembolism allows us to suspect the antiphospholipid syndrome (APS), and this in turn determines a different approach to secondary prevention and treatment. As thrombosis is a common event, laboratory diagnosis of LAC becomes crucial in identifying patients with APS.
When medium- to high-titer beta-2 glycoprotein I-dependent anticardiolipin antibodies and LAC are both present in the same patient’s plasma, a complete positive antiphospholipid profile may render physicians more confident with the diagnosis of APS. However, APS diagnosis becomes problematic, due to the abovementioned laboratory pitfalls, when LAC is the only positive test among those used to study antiphospholipid antibodies. Moreover, if antihuman prothrombin antibodies are responsible for LAC, they are poorly associated with thromboembolic events [31]. Therefore, the development of simple coagulation tests improving the clinical significance of positive LA is important [32].
Among the so-called antiphospholipid antibodies, the presence of LAC is strongly associated with thrombosis and obstetric complications. Thromboembolic complications, venous thromboembolism (deep vein thrombosis and/or pulmonary embolism), and cerebral ischemia (transient ischemic attack (TIA) or stroke) are the most frequent. In a systematic review of the literature, LAC has been shown to be statistically associated with both venous and arterial thromboembolism with an odds ratio ranging from 4.09 to 16.2. Obstetric complications include fetal death, preeclampsia or eclampsia, and multiple abortions. The association of other antiphospholipid antibodies such as anticardiolipin antibodies (aCL) and aβ2GPI with the clinical manifestations described above is less striking. The contemporaneous positivity in coagulation (LAC) and solid-phase assays (aCL and aβ2GPI antibodies) is due to the presence of a common pathogenic group of aβ2GPI antibodies.
As LAC is less sensitive with respect to solid-phase assays, triple positivity suggests that the aβ2GPI antibodies present in plasma are able to express LAC activity. In the absence of standardization and reference material for testing, an antiphospholipid laboratory profile could help to better classify these patients [33]. With this in mind we examined the results of all the three tests obtained in 618 consecutive subjects and compared subjects with previously documented thrombosis-related events with those without, for the most part, normal subjects. When antiphospholipid antibody profiles, instead of individual test positivity, were considered in a multivariate analysis taking into account age, gender, the presence of SLE or other autoimmune diseases, and established risk factors for venous and arterial thromboembolism, triple positivity was found to be a strong independent risk factor (odds ratio 33.3, confidence interval 7.0–157.6). Significance was maintained when an association with venous or arterial thromboembolism was considered. Double positivity with negative LAC was a highly significant risk factor for obstetric complications only (odds ratio 10.8, confidence interval 2.9–40.8). Other combinations were not statistically significant. The analysis of a complete antiphospholipid antibody profile, as compared to a single testing, can thus better identify patients at risk. In fact, it is a common experience that some isolated and often transient episodes of LAC positivity, such as those not infrequently found in children or young adults during a coagulation screening before surgery, are benign and not associated with thromboembolism. In the same way, isolated aCL positivity can easily be found in infectious diseases where no association with thrombosis has been reported. It is important to underline that only some antibodies to a specific domain of β2GPI express LAC activity and correlate strongly with thromboembolic events [34]. Other autoantibodies to β2GPI may not be pathogenic, and this may explain why studies on aβ2GPI antibody detection in solid-phase assays do not produce uniform results as IgG aβ2GPI is associated with thrombosis in only a subset of patients. In those cases (from 2 to 10 %) in which aβ2GPI is the sole antibody detected in patients with clinical manifestations of APS, it may not be pathogenic. In conclusion, a complete pattern of aPL antibodies comprising LAC, aCL, and aβ2GPI is important for identifying pathogenic aβ2GPI antibodies and the associated risk of clinical manifestations.
The prevalence of LAC in the normal population is reported to be 3.6 % according to screening using the KCT, but it may be much less. The prevalence of LAC in the SLE population has been reported to be between 10 and 50 %, depending on the test used [35].
The lupus anticoagulant is detected according to a set of diagnostic criteria recommended by the Scientific and Standardization Committee of the International Society of Thrombosis and Haemostasis. The sensitivity and specificity of these procedures vary, depending on the type of test, reagents, instrumentation, cutoff values, and the expression of results utilized. Many surveys have been carried out to evaluate the performance of clinical laboratories in LAC diagnosis. A recent survey was carried out within the framework of activities of the Italian Federation of Anticoagulation Clinics using affinity-purified lyophilized IgG aβ2GPI from a single patient. Overall, 69, 68, and 59 out of 70 participants were able to detect LAC in plasma with high, intermediate, and low potency (sensitivity 99, 97, and 84 %).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


