Task
Schizophrenia vs control
OCD vs control
Author
Deficits
Author
Deficits
ID–ED
Elliott et al. (1998)
More perseverative errors
Veale et al. (1996)
More failures at each stage
Pantelis et al. (1999)
More errors at ID and ED shift
Purcell et al. (1998a)
IDS lower score
Shamay-Tsoory et al. (2007)
More trials to reach criterion at ID reversal, ED shift and ED reversal
Chamberlain et al. (2006)
More trials to reach criterion on ED shift trials
Joyce et al. (2002)
75 % of patients were unable to perform an extra-dimensional shift
Purcell et al. (1998b)
No difference on IDS and EDS trial scores
Hutton et al. (1998)
First-episode schizophrenia patients had intact ability to switch attention
Nielen et al. (2003)
Require the same number of trials to complete the task, no difference in ID or ED errors
Tyson et al. (2004)
Fewer stages reached, more errors up to ED shift and at ED shift
Watkins et al. (2005)
More errors at ED stage, more patients failed to complete all stages
Jazbec et al. (2007)
Selective difficulties on C_D and EDS stages
Fenger et al. (2005)
Deficit in shifting attention, reversing response, performed more poorly on IDS and EDS trials
Braw et al. (2008)
More errors, fewer stages completed
Chamberlain et al. (2007b)
More trials to reach criterion on ED shift trials
Elliott et al. (1998)
More errors, more perseverative errors on ED and ID shift
Patel et al. (2010)
Marginally significant difference showing more errors in OCD patients for EDS stage
Ceaser et al. (2008)
More errors across all stages ID–ED; not shared with unaffected first-degree relatives
Yun et al. (2011)
More errors in acute schizophrenia, compared to remitted patients
SOC
Pantelis et al. (1997)
More moves, fewer perfect solutions, longer to execute, longer subsequent thinking time
Veale et al. (1996)
Longer generating solutions, more errors at each stage
Hutton et al. (1998)
Elliott et al. (1998)
Fewer correct on higher move problems
Longer initial movement time, longer subsequent movement time
Joyce et al. (2002)
More failures at ED stage, reached lower stages
Watkins et al. (2005)
One-touch TOL found intact planning ability
Tyson et al. (2004)
Solved fewer minimum move problems
Purcell et al. (1998b)
Longer, initial and subsequent movement times
Braw et al. (2008)
Longer initial thinking times, longer subsequent thinking times, solved fewer problems in minimum moves
Chamberlain et al. (2006b)
Lower strategy scores, generated fewer novel sequences after training
Pantelis et al. (1997)
Fewer perfect solutions, required more moves for completion, slower movement times and slower subsequent thinking latencies
Nielen and Den Boer (2003)
Fewer minimum move solutions, longer subsequent thinking time and longer time spent initiating and completing one sequence
Van del Heuvel et al. (2005)
More time generating alternative strategies following an incorrect move on the same task
Patel et al. (2010)
No significant difference
CGT
Hutton et al. (1998)
Fewer perfect solutions, total solutions and more moves per solution
Watkins et al. (2005)
Unimpaired on choosing the most likely outcome and OCD did not influence bet size or latency
Hutton et al. (2002)
Longer decision-making latencies, poorer quality of decision-making in chronic schizophrenia
Chamberlain et al. (2007a)
No difference on percentage of rational decisions made or percentage of points gambled
Patel et al. (2010)
No difference was found
AGN
No studies to date
Chamberlain et al. (2007a)
More omission errors for sad words
Watkins et al. (2005)
More false-positive errors following switch
Patel et al. (2010)
No difference was found
Patel et al. (2010) used tasks derived from the CANTAB to compare the performance of a group of clozapine-treated patients with schizophrenia alone to a group of IQ, symptom-severity and treatment-matched patients with schizo–OCD derived from the same clinical service. Patients were administered four domain-specific tests (see below), two of which were fronto-striatal tasks, derived from the CANTAB schizophrenia battery and chosen to evaluate executive functioning known to be affected in schizophrenia and OCD (Intra-dimensional–Extra-dimensional Set-Shift, Stockings of Cambridge). The additional tasks (Cambridge Gamble Task, Affective Go/No-Go task) measure aspects of motivational function and emotional processing, respectively, and tap ventral and medial–prefrontal (including orbitofrontal) areas of the cortex and the limbic and subcortical connections with this region. A detailed description of the tasks, rationale and results are provided below.
7.2.2.1 Intra- and Extra-Dimensional Set-Shift Task
The intra- and extra-dimensional (ID–ED) phases of the ID–ED set-shift test assess reversal learning and set-shifting, respectively. In humans, the former task is thought to depend upon the integrity of orbitofrontal neurocircuitry and the latter on ventrolateral prefrontal cortex circuits (Hampshire and Owen 2006). During the conceptually crucial extra-dimensional shift (EDS) stage, divergent thinking is required in order to shift attention away from a previously correct stimulus dimension to a novel (previously irrelevant) one. The total number of errors on the ID–ED and the number of errors at the EDS stage are used as the principal measures of attentional set-shifting (see Fig. 7.1).
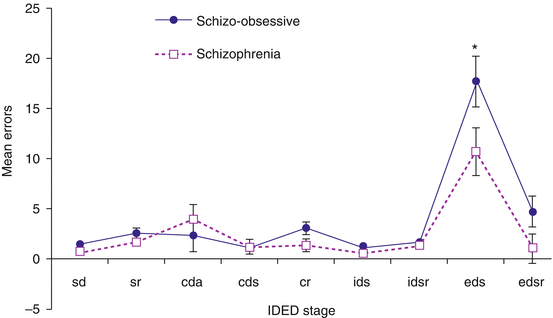

Fig. 7.1
Mean error at each stage of the intra-dimensional–extra-dimensional set-shift task, comparing schizophrenia with OCD (n = 12) and schizophrenia (n = 16) (Adapted from Patel et al. (2010). Cambridge University Press. Reproduced with kind permission of Cambridge University Press). SD simple discrimination, SR simple reversal, CDA compound discrimination adjacent, CDS compound discrimination superimposed, CR compound reversal, IDS intra-dimensional shift, IDSR intra-dimensional shift reversal, EDS extra-dimensional shift, EDSR extra-dimensional shift reversal, SE error bars
Compared with healthy controls, patients with schizophrenia exhibit significant deficits that include high levels of perseverative error on both elements of the task (Ceaser et al. 2008; Elliott et al. 1998; Tyson et al. 2004). Yun et al. (2011) showed that patients with non-remitted schizophrenia produced a significantly greater number of errors that occurred before the EDS (i.e. pre-ED errors) on this task, compared to remitted patients with schizophrenia and healthy controls (F = 5.6, p = 0.005). Interestingly, differences in the pre-ED errors and total adjusted errors on the ID–ED task became insignificant when scores on the Beck Anxiety Inventory were entered as the covariate, whereas other significant differences (e.g. stop signal reaction time and lower intelligence by WAIS) remained constant when these scores were entered, suggesting they reflect ‘state’ symptom severity and strengthening the importance of matching illness severity across schizophrenia groups when testing for set-shifting deficits. Importantly, in the study by Ceaser et al. (2008), the set-shift deficit seen in schizophrenia patients was not shared by unaffected relatives, again suggesting that this impairment represents the effect of illness state on performance and not an intermediate phenotype (endophenotype) of schizophrenia. In contrast, patients with OCD and their first-degree relatives show robust performance deficits, restricted to the EDS stage of the task (see Table 7.1) (Chamberlain et al. 2006a, 2007a; Veale et al. 1996; Watkins et al. 2005). Taken together, these results suggest that unlike in schizophrenia, in OCD, the cognitive inflexibility associated with EDS impairment represents a neurocognitive endophenotype.
Figure 7.1 shows the mean number of errors (and the standard error) at each stage of the ID–ED task in the study by Patel et al. (2010). Inspection of ID–ED errors at different task stages showed patients with schizophrenia and comorbid OCD separated from matched controls with schizophrenia at the two most difficult stages of attentional set-shift, i.e. EDS (extra-dimensional shift), in which the relevant stimulus dimension alters, and EDS reversal, in which a rule learnt needs to be inhibited and reversed. Although the error bars separated, when the correction for multiple comparisons was applied, the result for the EDS was marginally significant (F (1,26) = 4.03, p = .05). However, there was no significant difference between the number of errors on the extra-dimensional shift reversal (EDSR) stage between the two groups (F (1,26) = 2.96, p = 0.09). The EDS stage is the critical stage of the ID/ED and is thought to represent the category shift in the Wisconsin Card Sorting Test (Downes et al. 1989). The number of errors made at this stage represents attentional set-shifting ability. There was no evident relationship between ED impairment and schizophrenia or OCD symptom severity, and the deficits in those with schizo–OCD were present despite treatment with SSRI in 74 % of cases, implying trait rather than state-marker status and hinting that the EDS abnormality holds endophenotype status (see below).
7.2.2.2 Stockings of Cambridge (SOC)
The SOC assesses spatial planning and motor control. The task involves placing balls in sockets to match a given pattern within a specified number of moves. A low score on the measure ‘number of problems solved in minimum moves’ reflects inability to plan ahead. This task is thought to involve the dorsolateral prefrontal cortex and its subcortical connections in the dorsal striatum.
Patients with schizophrenia typically make fewer correct solutions and require more moves on the SOC task (see Table 7.1) (Braw et al. 2008; Elliott et al. 1998; Hutton et al. 1998; Pantelis et al. 1997; Tyson et al. 2004). In contrast, Purcell et al. (1998a, b) found that compared with controls, OCD patients spent more time engaged in movements on the SOC test, suggesting motor initiation and execution problems, but they showed no increase in thinking latencies nor increased error rates. Veale and colleagues (1996) reported that patients with OCD spend more time generating alternative strategies following an incorrect move on the same task. Similarly, Chamberlain et al. (2007a) found OCD patients made more attempts to correct a solution on the SOC task. A functional MRI study in patients with OCD using a version of the SOC found poorer planning, which was associated with decreased dorsolateral prefrontal cortex and caudate nucleus activity when compared with controls (van den Heuvel et al. 2005). However, no significant difference was found in performance of the SOC task when patients with schizophrenia and schizophrenia plus OCD were compared (Patel et al. 2010; p = 0.14), though the latter group showed poorer performance on this task.
7.2.2.3 Cambridge Gamble Task (CGT)
This task assesses impulse control, risk-taking and decision-making and is sensitive to the integrity of orbitofrontal neurocircuitry (Murphy et al. 2001; Rahman et al. 1999; Rogers et al. 1999). The participant is presented with a row of boxes, some of which are coloured red and others blue. Participants start by guessing whether a token is hidden in a red or blue box. In the gambling stages, subjects are given 100 points and can place a bet on the location of the token (either rising or falling offers) based on their confidence. The ratio of red to blue boxes is manipulated to present different levels of uncertainty of winning, and the aim is to accumulate as many points as possible. A higher score on the CGT risk-taking measure is indicative of more risk-taking. The task also measures the average latency to make a decision when placing bets.
Patients with schizophrenia showed longer decision-making latencies and poorer decision-making compared with healthy controls (Table 7.1; Hutton et al. 2002). Unlike patients with schizophrenia, however, studies in individuals with OCD do not usually demonstrate abnormal performance on the CGT (Chamberlain et al. 2007a; Watkins et al. 2005). In the study by Patel et al. (2010), no difference in CGT task performance was found between patients with schizophrenia with or without OCD (Table 7.1). Interestingly, however, in the schizo–OCD group, correlational analysis revealed that an increased severity of motor tic positively correlated with CGT decision latency (r = 0.65, n = 11, p = 0.03) (for further discussion, see later).
7.2.2.4 Affective Go/No-Go Task (AGN)
The AGN assesses information-processing biases for positive and negative stimuli and response inhibition (Murphy et al. 1999) and is sensitive to mood and orbitofrontal cortex function (Barnett et al. 2010). A series of words, either positive, negative or neutral, are rapidly presented. The participant is given a target category, e.g. negative, and is asked to respond whenever they see a word that matches the category. The AGN total omissions (e.g. failure to respond to sad words in sad word blocks) measure affective bias, whereas the total commissions measure response inhibition, where more errors would be indicative of motor impulsivity, i.e. an inability to inhibit motoric responses. Before the study by Patel and colleagues (2010), we are not aware of studies that used the AGN task in patients with schizophrenia. In contrast, patients with OCD are known to make more omission errors for sad words than matched controls, suggesting a selective attentional bias toward negative stimuli (Chamberlain et al. 2007a; Watkins et al. 2005). In the study by Patel et al. (2010), no significant difference was found between schizophrenia with and without OCD. Therefore, taking these results together with the CGT data, no between-group difference in performance appears to exist on tasks thought to probe ventral cortico-striatal circuitry function (GGT and AGN).
7.2.2.5 Other Potential Sources of Confound
Depressive symptoms are known to impair cognition and may confound interpretation of neurocognitive data. In the study by Patel and colleagues (2010), no correlation was found between level of impairment and OCD or depressive symptoms, consistent with neurocognitive impairment holding trait rather than state-marker status. Tic may represent another potential source of confound. In the same study, patients with schizo–OCD exhibited a trend toward increased motor tic, emphasising a neurological contribution to schizo–OCD (M = 9.92, SD = 15.08, p = 0.04). Furthermore, motor tic was related to longer decision latency on the CGT. This single significant correlation might simply reflect a chance finding. However, considering the results from the study by Watkins et al. (2005), in which patients with Tourette’s syndrome showed greater impairment on decision-making tasks, compared with OCD and normal controls, such a result may implicate longer decision latency on the CGT as a neurocognitive marker related to the presence of motor tic.
7.3 Neurocognitive Endophenotypes in Schizo–OCD
Patients with schizo–OCD appear to exhibit significantly greater impairment in executive function compared to patients with schizophrenia, and this dysfunction is particularly apparent in tests of cognitive flexibility such as the category shift of the WCST and the EDS of the ID–ED task. The specific EDS deficit found in the small study by Patel et al. (2010) merits further validation, as all patients were antipsychotic resistant and were taking clozapine. Therefore, the result cannot necessarily be generalised to all cases of schizophrenia.
Evidence from studies in unaffected first-degree relatives of tic-free OCD patients with predominantly washing and checking rituals suggests that EDS impairment may represent a neurocognitive endophenotype (Chamberlain et al. 2006a, 2007b). In the study by Chamberlain et al. (2007b), non-affected relatives of OCD probands exhibited the abnormality in EDS impairment, which was unrelated to symptom severity and endured despite treatment, suggesting EDS represents a trait marker (familial vulnerability marker, unrelated to state illness or medication confounds) reflecting genetic vulnerability to OCD. Chamberlain et al. (2007b) proposed that the inability to shift attentional focus may result in functional cognitive inflexibility and contribute to the generation of compulsive symptoms.
Furthermore, EDS deficits are found to occur across a range of compulsive disorders and may underpin the generation of compulsive symptoms across traditional diagnostic groupings. For example, impaired EDS has been identified in patients with obsessive–compulsive personality disorder (Fineberg et al. 2007) and body dysmorphic disorder (Jefferies et al. 2012; Labuschagne et al. 2013). We may therefore extend this hypothesis to propose EDS cognitive inflexibility as a candidate endophenotype for schizo–OCD; replication of this result in the unaffected relatives of patients with schizo–OCD would indicate (a) that this association is not merely state related and (b) that there may be a shared genetic vulnerability for both schizophrenia and OCD in this grouping (Poyurovsky and Koran 2005). Further support could therefore be derived from further investigation of the non-affected first-degree relatives of patients with schizo–OCD. So far, the only study to investigate cognitive inflexibility in the unaffected relatives of patients with psychosis used a set-shift task (Response Shifting Task) that was unable to discriminate the psychotic patients with OCD from those without (Meijer et al. 2013). However, regression analysis indicated that in the unaffected parents, the severity of subclinical OCSs (Y-BOCS score) was significantly correlated with poorer set-shifting ability (based on Response Shifting Task accuracy), although the effect size was small (d′ = −0.10). Thus, in individuals with increased vulnerability to psychosis, there is some weak evidence that impaired set-shift performance may be associated with the presence of OCS.
The evidence of trans-diagnostic impairment in cognitive flexibility that characterises a range of compulsive disorders and that may extend to schizo–OCD suggests that in schizo–OCD, a form of ‘true’ overlap exists (rather than schizo–OCD representing a subset of schizophrenia or an artefact of treatment). This raises further important questions, such as: What is the mediating neurobiology? Are there treatment implications? Could a wider range of tests identify additional behavioural features unique to schizo–OCD?
7.4 Probing the Mediating Neuropsychology and Treatment Implications
According to studies using fMRI in healthy subjects, ‘cognitive flexibility’ or ‘cognitive inhibition’ as measured by EDS performance is selectively associated with activation in the circuits connecting the bilateral ventrolateral prefrontal cortex with subcortical structures (Hampshire and Owen 2006). Using another task-switching fMRI activation paradigm, Gu and colleagues (2008) investigated a group of 21 patients with OCD compared with 21 healthy subjects of matching age, IQ and sex. In the healthy subjects, the task-switch produced activation in widespread networks both in cortical and subcortical brain regions including the fronto-striatal circuit, which was reduced significantly in OCD patients during the same task. Significant between-group differences were also observed in the ventromedial prefrontal and right orbitofrontal cortices. According to the authors, these findings provide neural correlates related to a task-switching deficit in OCD and suggest that impaired task-switching ability in OCD might be associated with an imbalance in brain activation between dorsal and ventral fronto-striatal circuits (Gu et al. 2008). However, compared with the healthy subjects, patients with OCD exhibited a significantly higher error rate in task-switch trials (p < 0.05), which may have introduced a source of confound in the imaging results. Indeed, correlational analysis indicated that the activation of orbitofrontal cortex was related to task performance in both groups and also with the activation of anterior cingulate cortex in the OCD group.
While fMRI has localised certain brain regions involved in set-shifting, it does not capture the millisecond timing of neurocognitive processes. Magneto-encephalography (MEG) is a modality that provides improved temporal resolution for examining the timing of activations across brain regions during cognitive tasks. A recent study (Oh et al. 2014) that explored the location and timing of frontal and parietal activations during an ID–ED task found that activations related to EDS were detected in left inferior frontal gyrus, left middle frontal gyrus and right middle frontal gyrus between 100 and 350 ms, followed by superior frontal gyrus between 250 and 500 ms. EDS also activated right parietal areas – areas previously implicated in OCD (Chamberlain et al. 2008; Oh et al. 2014).
Pharmacological probe work in healthy volunteers suggests that the EDS can be modulated by compounds with dopamine receptor antagonist effects (Mehta et al. 1999). Patients with schizo–OCD are usually receiving treatment with dopamine antagonist agents, which therefore may contribute toward this problem. Multiple lines of evidence also implicate glutamate signalling in the dorsal striatum as a mechanism for regulating behavioural flexibility. Animal research implicates interaction between the striatal metabotropic glutamate receptor (mGlu5R) and the ionotropic N-methyl-D-aspartate receptor (NMDAR) in modulating aspects of cognitive flexibility in instrumental learning, based on the contingency-dependent attribution of goal-directed versus habitual activity (Lovinger 2010). Modulation of the mGlu5R in rats using a selective positive allosteric modulator has been shown to produce beneficial effects in the treatment of perseverative and set-shifting deficits (Gastambide et al. 2012). In another study, modafinil, a central nervous system wakefulness-promoting agent, was found to remediate the EDS decrement in patients with schizophrenia (Turner et al. 2004). The mode of action of modafinil remains somewhat unclear, but its cognitive-enhancing properties might result from glutamatergic and/or dopaminergic increased neuronal activation in the hippocampus and in the prefrontal cortex, respectively (Scoriels et al. 2012). Investigation of new treatment targets, such as the pharmacological manipulation of dopamine, NMDAR or mGluR function in disease states, might be of particular relevance in remediating cognitive inflexibility in schizo–OCD and the associated severe executive dysfunction associated with the disorder.
Cognitive inflexibility negatively impacts upon evidence-based treatment for compulsive disorder such as CBT and may partly explain the relatively treatment-resistant nature of schizo–OCD (reviewed in Mukhopadhaya et al. 2009; Schirmbeck and Zink 2012). Often, it is the compulsive symptoms themselves that become the most intransigent and impairing problem for patients with schizo–OCD (see our vignette). New treatments targeting cognitive inflexibility are under investigation in compulsive disorders such as OCD and eating disorder and have yielded promise of efficacy in small trials, with potential to improve treatment outcomes. These include psychological (cognitive remediation therapy (CRT)) and brain stimulation strategies (repetitive transcranial magnetic stimulation (rTMS)).
CRT has been developed for and tested in eating disorder, targets set-shifting, helps patients adopt flexible behaviour strategies, increases motivation and perceived ability to change and enhances success when combined with usual forms of treatment. Two small-scale trials in OCD employing CRT were effective in improving compulsive symptoms as well as executive skills and cognitive flexibility, respectively (Buhlmann et al. 2006; Park et al. 2006). A recent randomised controlled trial (RCT) in eating disorder showed individual CRT given with CBT improved quality of life and dropout rates compared with CBT alone. Poor baseline EDS predicted a better response to CRT (Dingemans et al. 2014). These results strongly suggest that individual CRT may be a beneficial adjunct to usual treatment in other compulsive disorders characterised by cognitive inflexibility, such as schizo–OCD.
Repetitive (r)TMS has produced variable results in compulsivity. In OCD, rTMS of the dorsolateral PFC was not found to be effective, though two small RCTs have reported promising results for low-frequency rTMS applied to the pre-supplementary motor area and the orbitofrontal cortex, respectively (Jaafari et al. 2012). Better targeting of fronto-striatal neurocircuitry, using coils that can induce stimulation in deeper brain areas, higher stimulus intensities and longer treatment course (3–6 weeks), has been suggested as a means to improve therapeutic outcome. Consequently, TMS studies using deep H-coils are ongoing in several disorders of compulsivity and are starting to produce positive results for OCD and addiction (Carmi et al. 2013; Dinur-Klein et al. 2014). There would be grounds to consider the future investigation of rTMS or deep TMS as an adjunct to usual treatment in patients with schizo–OCD.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


