Fig. 4.1
The mouse uterus shows distinct endometrial (E), inner circular (IC) and outer longitudinal (OL) myometria layers that are separated by a loosely packed vascular layer (V) (haematoxylin and eosin)
Fig. 4.2
Haematoxylin and eosin section of the human myometrium showing the lack of distinct muscle layers
There is growing evidence for the role of myometrial stem cells particularly in relation to uterine growth in pregnancy [11]. Clonal smooth muscle and fibroblast proliferation has been shown in relation to fibroids [12] but the relevance of this to regional myometrial zonation or to the development of adenomyosis remains speculative.
The Inner Myometrium or Myometrium Junctional Zone
There is evidence that the inner myometrium develops earlier in utero and is visible before week 21 of gestation compared to the outer myometrium which only appears after 24 weeks of gestation [13]. In contrast to the well-defined circular and longitudinal layers found in rodents (Fig. 4.1) and in many other species, the microanatomy of the human uterus is complex. Confusion arises from the observation that the subserosal myometrium appears to be different to the inner myometrium. The outer myometrium contains large blood vessels with their own smooth muscle cell coat and sensory neurones that are under hormonal influence. The question arises as to whether there are distinct functions for the inner and outer myometrial layers and how the two layers are differentially regulated or affected by the presence of commonly encountered diseases such as adenomyosis and fibroids. The inner myometrium close to the endometrial junction exhibits higher cellular density compared to the outer myometrium and also higher nuclear area [14], and that the transition from the inner to the outer myometrium is gradual with no clear demarcation [14].
It is thought that the inner myometrium is responsible for supporting sperm transport and the retention of the blastocyst prior to implantation. The inner myometrium may also have a role in aiding the separation of endometrial functionalis [15] and possibly in the regulation of uterine blood loss during menstruation [16].
In rodent pregnancy the two muscle layers have clear and different roles. The circular muscle is responsible for maintaining the fetal units as separate entities and assisting with parturition [17] whilst the longitudinal muscle aids the correct positioning of the fetal units within the uterus and retraction of the cervix towards the oviducts during parturition.
The Myometrium and Sperm Transport
It has been suggested that the inner myometrium of the non-pregnant uterus (in rodents and human) aids sperm transport from the region of the cervix [15, 18]. This may be triggered by prostaglandins and other inflammatory mediators in the ejaculate. The resulting sporadic contractions of the inner myometrium create negative intra-uterine pressures that draws the sperm into the uterine cavity where it binds to pinopodes on endometrial epithelial cells [19]. Sexual stimulation and the release of oxytocin results in more coordinated and forceful uterine contractions creating a peristaltic wave from the cervix towards the oviduct and these contractions position the sperm closer to the oviduct where a relatively small number migrate for fertilisation to take place [20].
An alternative mechanism postulates that, in the female, an intrinsic contractile feedback response during intercourse increases the stimulation of the purinergic and neurotrophin-sensitive neurones that innervate the outer myometrium. This, in turn, stimulates the entire uterus to contract and thus move sperm towards the oviduct. Simultaneously, the release of mediators associated with ‘pleasure’ stimulates the basal cilial receptors which aid sperm progression in the oviduct from the isthmus to the ampulla. Endocannabinoids, which increase ciliary beat frequency and sperm motility in vitro, may have a role in this process [21]. A similar mechanism may be involved in retrograde uterine transport of endometrial tissue during menstruation and this may be a factor in the pathogenesis of endometriosis [22].
The Myometrium During Pregnancy
The uterus is a unique organ in that its primary role of holding and nurturing the developing fetus is concerned with the preservation of the species rather than the survival of the individual. For pregnancy to be successful, the process requires coordinated sequential changes. The changes that occur in the myometrium during the earlier synthetic stage of pregnancy are relatively slow when compared to the changes associated with the contractile stage that occurs at the end of pregnancy and during the perinatal period.
Synthetic Stage
The uterus adapts to all stages of fetal development from implantation to term. In the rodent, fluctuations in muscle tension in the outer longitudinal muscle layer space the blastocysts along the length of the tubular uteri [23], a process that may be influenced by blastocysts. Mediators, such as the lipid-like substance released by blastocysts that inhibits the local expression of the endocannabinoid catalytic enzyme fatty acid amide hydrolase (FAAH) at the implantation site [24] leading to an increase in endocannabinoids at the implantation site are suggested to play a key role [25]. Myometrial receptors for endocannabinoids inhibit myocyte contraction [26, 27], suggesting that focal relaxation of the smooth muscle fibres at the point of implantation but relatively higher levels of contraction between implantation sites assist in the correct positioning of the blastocysts along the length of the uteri. In species that are confined to mainly singleton pregnancies, evidence for the induction of FAAH or decrease of endocannabinoids at the site of implantation is currently lacking [28].
The human uterus enlarges from approximately 75 g before pregnancy to approximately 1300 g at term. The increase in uterine weight occurs through myocyte hypertrophy rather than hyperplasia during the ‘synthetic stage’ of myometrial development. During this stage, the myometrium remains refractory to contractions and the enzyme protein kinase Cα (PKCα) may have a role in myometrial quiescence [29]. The key hormone involved is progesterone, which binds to progesterone receptors (PR) affecting transcription regulation [30]. PR-B dominates in the human myometrium during pregnancy, whilst the PR-A dominates in the decidua [31]. Progesterone decreases proinflammatory gene expression when the PR-A to PR-B ratio favours PR-B and increases proinflammatory gene expression when the ratio favours PR-A. This mechanism of action has been shown to be mediated by PR-B leading to increase in the expression of inhibitor- κΒα, a repressor of the nuclear factor-κΒ transcription factor, and inhibition of basal and lipopolysaccharide-induced proinflammatory gene expression. At parturition, the rise in PR-A expression promotes labour by inhibiting the anti-inflammatory actions of PR-B and stimulating proinflammatory gene expression in response to progesterone [32].
Contractile Stage
An important function of the myometrium is to respond to contractile signals in labour. This phase is characterised by the up-regulation of contraction-associated proteins, such as the gap junction proteins, connexin 43 and 45, oxytocin receptor, and the prostaglandin synthesis enzyme cyclooxygenase [33].
Uterine contractions leading to delivery involve coordinated smooth muscle cell contraction and retraction followed by involution to the prepregnancy state. Labour-associated proteins are influenced by biomechanical stretch [34, 35], which during the period of fetal growth, remain low, only increasing in response to a critical ‘limit of stretch’ that triggers the expression of connexin 43, cyclooxygenase-2 and the oxytocin receptor [36, 37]. It has been shown that biomechanical factors rather than the presence of the fetus are responsible for the production of the contraction-associated proteins [38]. Similar increases in some of these proteins have been demonstrated in cultured human myocytes [39]. One unifying theory for myometrial response is based on the observation that up regulation of the connexin 43 protein could induce a syncytial myometrium that is able to respond to contractile stimuli in such a way that the entire uterus contracts as one.
The final major function of the myometrium during pregnancy occurs after the fetus has been delivered as the uterus undergoes tonic contraction to shear and expel the placenta with the attached fetal membranes. Failure of this stage can lead to post-partum haemorrhage. Subsequent uterine involution occurs over approximately 4–6 weeks, with the initial stages reducing myocyte size and number through both necrosis and apoptosis by mechanisms that include the recruitment of immune cells and autophagy [40]. This is followed by a small increase in new myocytes. Currently, the initial increase in cell number is considered to result from either an expansion of stem cell [41] or existing myocyte populations [40].
The Impact of Adenomyosis
Health information websites report that during the normal menstrual cycle most patients with adenomyosis have no outward symptoms, and can pass their entire reproductive life with asymptomatic adenomyosis, whilst the medical literature reports that only 35–40 % of women are asymptomatic [42]. Adenomyosis can be associated with a range of concomitant pathology including endometriosis and fibroids. The peak incidence of adenomyosis occurs in the fourth decade of life [43] and many women with adenomyosis also have endometriosis, which may itself account for symptoms of dysmenorrhoea, infertility and possibly menorrhagia. There is some evidence of heightened central nervous system sensitivity in some women with adenomyosis, resulting in heightened pain perception, presumably because the myometria of women with adenomyosis have increased numbers of sensory neurones. This increase in neuronal density is dependent upon local production of neurotrophins and their cognate receptors [44] and an altered expression of the neurotrophins NGF and BDNF and their cognate receptors have been reported in the myometrium in women with adenomyosis [45, 46]. This is in line with previous reports of increased nerve growth factor expression and changes in the lower affinity neurotrophin receptor (p75NTR) in the mouse model of adenomyosis [47].
PGP9.5 immunoreactivity was identified in the functional layers of the endometrium in women with adenomyosis and fibroids who also had pain symptoms but not in women without pain [48]. PGP9.5 nerve fibre density in the basal layer of the endometrium and in the myometrium was also significantly increased in women with pain [48]. It was hypothesised that dysmenorrhoea linked to adenomyosis may result from bleeding within the adenomyotic nodules in response to steroid withdrawal at menstruation, although entrapped blood is not commonly observed in affected uteri. Many of the ligands and receptors for the neurotrophin family of proteins have been shown to increase under the influence of oestradiol [49] which has been implicated in the aetiology of adenomyosis [47].
If the propagation of myometrial contractility and function is dependent on the production of a complete syncytium, then the presence of adenomyotic lesions may affect the normal physiology of the myometrium by interfering with the transfer of small molecules through the gap junctions produced by connexin proteins or by interrupting the propagation of membrane depolarisation through the muscle fibres. In women with adenomyosis, this might impair sperm transport [50], reduce fertility [51, 52], increase first trimester miscarriage [52, 53], increase the incidence of preterm labour [54] and precipitate placental abnormalities [55]. Recent research has found that women with adenomyosis undergoing IVF/ICSI treatment for infertility have higher miscarriage rates than women without the condition, with lower pregnancy rates and a much higher risk for early pregnancy loss [54]. Affected women also appear to have an increased risk of premature labour and placental abruption [55]. This suggests a dysfunctional myometrium. Another serious but rare complication of adenomyosis in pregnancy is the risk of uterine rupture during labour [56]. This may be due to aberrant decidualisation of deep lesions resulting in a reduced myometrial mass leading to rupture under tension. However, the majority of women with adenomyosis do become pregnant with normal outcome, suggesting that sperm transport may not be significantly affected by adenomyosis. But fertility rates measured by on-going pregnancy (but not biochemical pregnancy) in assisted reproduction are lower in adenomyosis [57], suggesting that impairment of blastocyst retention rather than in sperm delivery and fertilisation occurs in these women.
Whether there is altered decidualisation in adenomyosis remains uncertain [58]. Preliminary data demonstrated no impairment of in vivo decidualisation of stromal cells derived from adenomyosis as evidenced by normal production of prolactin and insulin-like growth factor binding protein-1 (IGFBP-1) (Fig. 4.3). It is possible that lower on-going pregnancy rates results from inner myometrial hyperperistalsis or dysperistalsis [59], resulting in disruption of implantation, perhaps under the influence of the increased production of nitric oxide [60].
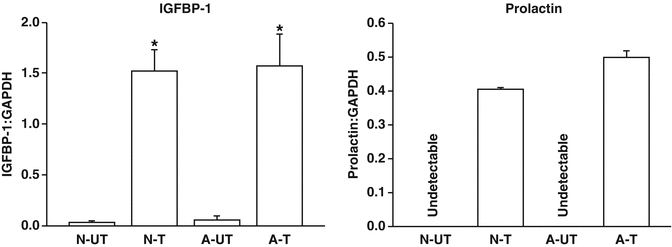

Fig. 4.3
Effect of adenomyosis on in vitro decidualisation markers. Stromal cells derived from adenomyotic (A) and normal (N) endometria were subjected to in vitro decidualisation in the presence of 17β-oestradiol (10−8 M), 8-Bromo-cyclic AMP (0.5 × 10−3 M) and medroxyprogesterone acetate (10−6 M) for 21 days (T) or with 0.1 % ethanol (UT), with medium changes occurring on alternate days. Cellular mRNA was incubated with AMV-RT and the resultant cDNA subjected to PCR. The levels for IGFBP-1 and prolactin transcript were corrected for that of GAPDH. Data are presented as the mean ± SEM of three biological replicates performed in triplicate. The number of independent data points (n) thus equals nine. Comparison of IGFBP-1 levels in the decidualised adenomyotic and normal stromal cells against the untreated controls was performed with Student’s t-test; *p < 0.05. Prolactin transcript levels in the untreated stromal cells were undetectable
Ectopic pregnancy rates may not be increased in women with adenomyosis suggesting normal transport of the zygote through the oviduct, but ectopic pregnancy has been linked to adenomyosis in smokers [61] and a case was reported of an intramural pregnancy linked to adenomyosis [62], although such cases must be extremely rare. Caesarean section rates are not significantly increased in women with adenomyosis and affected women do not experience prolonged labour [61], however, myometrium affection varies significantly depending on the extent of the disease and further research is needed to correlate disease spread and its impact.
Conclusion
Despite recent technological advances, there remains a paucity of information on many important aspects of uterine physiology and on the impact of adenomyosis. Even though research on the functioning of the myometrium in pregnancy is moving at a steady pace, our understanding of the role of the myometrium during the non-pregnant state remains limited.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


