CHAPTER 50 The mechanism of continence
Urinary Incontinence
Anatomy of the lower urinary tract
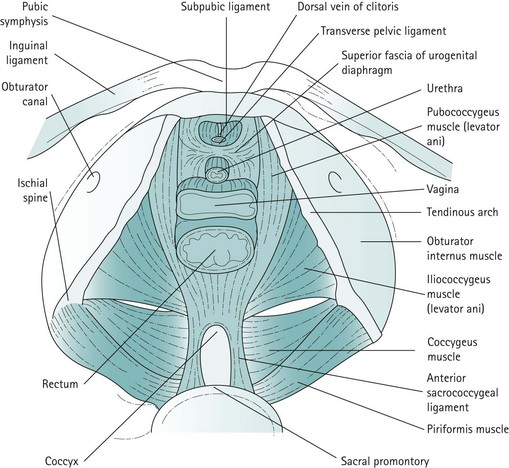
The pelvic floor
For the context of this chapter, the pelvic floor structures that will be described are the levator ani muscles, the endopelvic fascia and condensations of this fascia which form the ligaments. The levator ani muscles are often described as funnel-shaped structures, but in-vivo imaging has revealed that they are, in fact, more horizontal. The fibres of each side pass downwards and inwards. The two muscles, one on each side, constitute the pelvic diaphragm. Defects in the levator ani allow the urethra, vagina and rectum to pass. The levator ani is subdivided into the pubococcygeus, iliococcygeus and coccygeus. The pubococcygeus arises anteriorly from the posterior aspect of the pubic bone and from the anterior portion of the arcus tendineus (white line), which is a condensation of the obturator internus fascia. The medial fibres of the pubococcygeus merge with the fibres of the vagina and perineal body, and have been given various names such as puborectalis, pubourethralis and pubovaginalis. The iliococcygeus arises from the remainder of the arcus tendineus, partly overlapping the pubococcygeus on its perineal surface and extending to the medial surface of the ischial spine. The coccygeus or ischiococcygeus is a rudimentary muscle arising from the tip of the ischial spine, and quite often constitutes only a few muscle fibres on the sacrospinous ligament. Posteriorly, the muscles of the levator ani or pelvic diaphragm insert into the sides of the coccyx and the anococcygeal raphe, which is formed by the interdigitation of muscle fibres from either side. The most medial fibres of the pubococcygeus that pass round the rectum at the anorectal junction form the puborectalis muscle (Figure 50.1).
Innervation of the bladder and urethra
The bladder has a rich parasympathetic nerve supply (Figure 50.2). The postganglionic cell bodies lie either in the bladder wall or pelvic plexuses, innervated by preganglionic fibres that originate from cell bodies in the grey columns of S2–4. There is little sympathetic innervation of the bladder, although greater quantities of noradrenergic terminals can be detected in the bladder neck or trigone. Noradrenergic effects can be either inhibitory or excitatory, depending on the receptor type present.
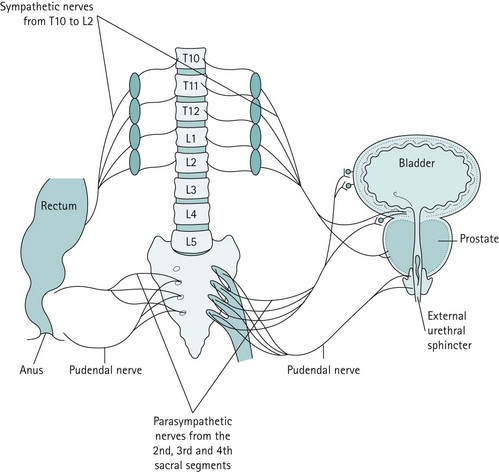
Figure 50.2 The major innervation pathways at the pelvic level that are basic to control of micturition and continence.
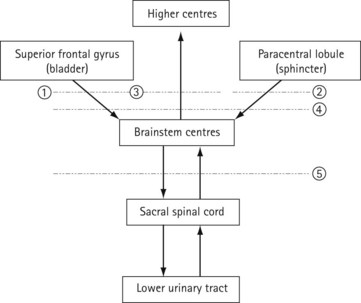
Central nervous control of continence
The connections of the lower urinary tract within the central nervous system are complex (Figure 50.3). There are many discrete areas which influence micturition, and these have been identified within the cerebral cortex, in the superior frontal and anterior cingulate gyri of the frontal lobe and the paracentral lobule; within the cerebellum, in the anterior vermis and fastigial nucleus; and in subcortical areas, including the thalamus, the basal ganglia, the limbic system, the hypothalamus and discrete areas of the mesencephalic pontine medullary reticular formation. The full function and interactions of these various areas are incompletely understood, although the effects of ablation and tumour growth in humans and stimulation studies in animals have given some insights.