Non-reassuring preterm cardiotocographic trace. A nulliparous woman presented with contractions and spontaneous rupture of the membranes with maladorous amniotic fluid at 34 + 2/7 weeks’ gestation. The cervical dilatation was 4–5 cm and the fetus was presenting cephalically with the presenting part above the ischial spines. Electronical fetal monitoring was established and at 04:45 h (arrow 1 on CTG trace) an intravenous injection of 0.25 mg of terbutaline was given for acute tocolysis to correct tachysystole and a non-reassuring CTG trace (complicated variable decelerations). Ten minutes later a fetal scalp blood sample (FBS – arrow 2) was performed with a pH of 7.17. An emergency caesarean section was carried out. The baby was born with a birth weight of 1960 g, with Apgar scores of 51 and 85 and umbilical cord artery pH of 7.07 and standard base excess of 12.6.
Mode of Delivery of the Preterm Infant
In both term and preterm deliveries, vaginal delivery (VD) should be the preferred route as it is associated with lower maternal morbidity and mortality. The reason for complications after preterm caesarean section (CS) is uncertain, as it is often difficult to determine whether it is the CS per se or the underlying indication for the CS that causes the complication. At early gestations, the lower segment of the uterus is poorly formed or undeveloped. Accordingly, at the time of preterm CS it may be necessary to perform a low classical uterine incision or a T- or J-incision on the uterus. The higher maternal risks of planned CS compared to VD are shown in Table 21.2. There are a number of factors that influence the decision with respect to mode of delivery of the preterm infant and some of these are listed in Table 21.3. Since the situation may change rapidly and there are two patients to consider, time may be limited to make optimal decisions. Finally, around the limits of viability (22–26 completed weeks of gestation) the decision may be particularly problematic and full discussion with the parents should involve the neonatal paediatricians. There are fetal risks of CS compared to VD. A poorly formed lower uterine segment may cause technical difficulties when delivering the infant. The baby’s head is particularly vulnerable, with a risk of intracerebral haemorrhage as well as abdominal visceral injuries and injuries to the limbs and skin. Accordingly, one method of delivering the preterm infant by CS to protect against such injuries is birth ‘en caul’, where the fetus is delivered within an intact amniotic sac (Figure 21.2). With elective CS without labour there is the additional risk of transient tachypnoea of the newborn, as well as idiopathic respiratory distress syndrome. Furthermore, there is a risk of iatrogenic prematurity where a planned CS may be a consequence of misdiagnosed labour (see above). Accordingly, a policy of planned CS for women in SPTL carries a higher risk of increasing numbers of PTBs. To date, only six RCTs to determine the optimum mode of delivery for the very preterm infant have been performed and all six trials were stopped early due to small numbers. These trials did not yield enough evidence to evaluate the use of planned CS vs. VD in SPTL. However, seven cases of major maternal postpartum complications in the CS arm were found compared to none in the VD arm in four of the trials (RR 7.21; 95% CI 1.37–38.08) [27].
|
|
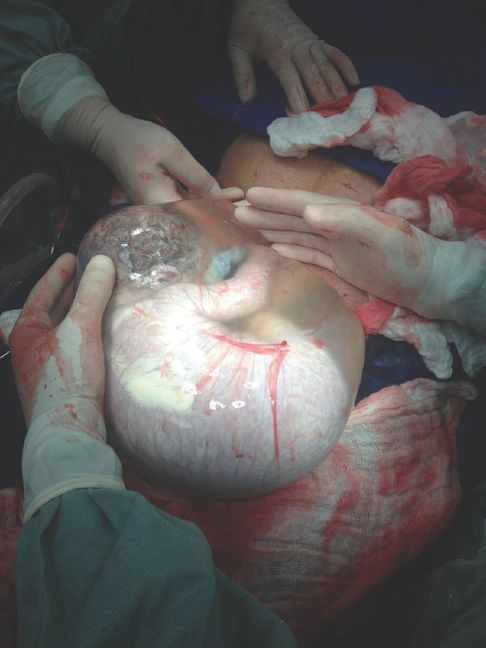
Delivery of the preterm infant ‘en caul’. Caesarean section with ‘en caul’ delivery of the preterm infant within an intact amniotic sac with gentle and blunt removal from the uterine cavity to prevent pressure trauma to the head, abdominal viscera or injuries to the limbs and skin of the infant (with kind permission from Dr Aris Tsigris (www.aristsigris.gr/#). For a video file of an ‘en caul’ delivery pleae visit www.cambridge.org/9781107472341.
Mode of Delivery of the Preterm Infant Presenting Cephalically
In general, VD for the preterm infant presenting cephalically is preferred, but CS can be justified for feto-maternal indications. Based on empirical data, for women presenting in SPTL with a cephalic presentation before 24 completed weeks of gestation, CS for a fetal indication is normally not justified. Between 24 and 26 completed weeks of gestation, CS for a fetal indication can be justified and after 26 completed weeks of gestation, CS for a fetal indication is always justified.
Mode of Delivery of the Preterm Infant Presenting by the Breech
For the preterm infant presenting by the breech, the risk of fetal complications after VD appear to be higher and hence a CS is often preferred. Before 32 completed weeks of gestation and/or with breech presentations with an estimate of fetal weight of <1500 g there is a substantial body of retrospective, descriptive literature that supports CS due to reduced neonatal mortality and morbidity. The risks pertaining to VD include a high incidence of footling breech presentation with greater likelihood of cord accidents and increased risk of asphyxia. Furthermore, there is a higher risk of entrapment of the after-coming head through an incompletely dilated cervix. Infant mortality and morbidity following delivery of the preterm infant presenting by the breech is inversely proportional to gestational age; several (older) reviews confirm this [28]. Large registry studies from the Nordic countries support these findings and report that VD of preterm infants is associated with a small but increased risk of infant death in breech and multiple births. However, in VD of vertex presentations there was no increased risk (excluding cases with pre-eclampsia) [29].
Vaginal Operative Deliveries of the Preterm Infant
It is generally accepted that vacuum extraction (VE) should not be performed before 34 weeks’ gestation due to the higher risk of cephalhaematoma and intracranial haemorrhage. The use of forceps or episiotomy does not improve outcome. There are very few studies, most of which are inconclusive, on neonatal outcome after VE for PTB. The conclusions are that, in general, the rate of VE in PTB is very low but is associated with a higher risk of intracranial and extracranial haemorrhage and brachial plexus injury. This is in contrast to CS during SPTL compared with VD, in which there was no increased risk of these outcomes. The authors called for continued caution with use of VE for PTB, especially before 34 weeks’ gestation [30].
Preterm Prelabour Rupture of the Membranes
Approximately one-third of all PTBs are preceded by pPROM, which is defined as rupture of the fetal membranes prior to 37 weeks’ gestation before the onset of contractions. pPROM complicates only 2% of pregnancies but can result in significant neonatal morbidity and mortality mainly due to sepsis, immaturity and pulmonary hypoplasia. Accordingly, the management of pPROM is a balance between expectant management and the risk of infection versus early intervention and the risk of iatrogenic prematurity. The management of pPROM requires accurate diagnosis and will depend on gestational age. At term, prelabour rupture of the membranes usually results in spontaneous labour within 24 hours. In contrast, in 75% of pPROM before 28 completed weeks of gestation, labour has not started by one week. Traditionally, the diagnosis of pPROM has been clinical (detection of the characteristic pool of liquor on sterile speculum examination), ultrasonographic (oligohydramnios in association with a good history of pPROM), biophysical (the nitrazine test to detect pH change) and microscopic (characteristic crystalline ferning pattern of dried amniotic fluid). Recently, a biochemical method has become commercially available which comprises a rapid immunoassay for placental α-microglobulin-1 with a sensitivity and specificity of 98.9% and 100% respectively for the diagnosis of ruptured membranes. pPROM before 34 completed weeks of gestation is normally managed expectantly with the use of antibiotic prophylaxis and administration of a full course of APGs. The feto-maternal benefits of prophylactic antibiotics for the management of pPROM are well recognized, but the choice of antibiotic is still being debated. Following the findings of the ORACLE I study, most units administer prophylactic erythromycin, but this does not provide cover against many significant pathogens such as mycoplasmas and anaerobes, for which the addition of clindamycin should be considered. Between 34 and 37 completed weeks of gestation, obstetricians differ in their approach to pPROM. Some feel that the benefits of greater maturity outweigh the risks of infection and so follow expectant management. While birth between 34 and 37 completed weeks of gestation is associated with less neonatal mortality and morbidity, there is still a burden of death and handicap for PTBs during this period. Others feel that the risks of infection outweigh the risks of prematurity and so, if labour has not ensued within 48 hours, or when the course of APGs has been completed, they will induce labour and delivery.
Management of SPTL at the Limits of Viability
Around the limits of viability (22–26 completed weeks of gestation) the risk–benefit balance of some interventions may change. The use of interventions in which the risks of adverse events outweigh the theoretical benefits at 34 weeks’ gestation may be considered at 24 weeks’ gestation in a heroic attempt to improve outcome. Clearly a balance has to be reached between therapeutic nihilism and reckless imprudence, but there is some support for the beneficial use of combined interventions around the limits of viability that would not be considered at later gestations [31]. Parents should know what to expect with respect to obstetric interventions such as CS or neonatal resuscitation. It is therefore vitally important to ensure that midwives, obstetricians and neonatal paediatricians communicate with each other and that the resultant information to the parents on obstetric/neonatal intervention or no intervention is made perfectly clear.
Magnesium Sulphate as a Neuroprotective in the Management of PTB
While the rate of PTB is increasing [32] and the survival rate has improved, especially at very early gestations, the prevalence of cerebral palsy (CP) has increased. Since the incidence of CP is inversely related to gestational age, it would seem obvious to implement treatment strategies to reduce the risk of CP at early gestational ages. Some 15 years ago it was demonstrated that the outcome of infants born to mothers treated with magnesium sulphate, either as a tocolytic or for prophylaxis against eclampsia, caused a reduction in the rate of CP. This putative neuroprotective effect of magnesium sulphate was then demonstrated in RCTs and meta-analyses [33] where intravenous magnesium sulphate was compared to placebo in women at risk of PTB. A Cochrane review from 2009 found enough evidence to conclude the same, with a 32% reduction of CP (RR 0.68; CI 0.54–0.87) [34]. However, despite national guidelines from Australia, Canada and the UK which conclude that antenatal magnesium sulphate reduces the risk of CP in preterm infants, most obstetric departments have not implemented this as standard treatment. The American College of Obstetricians and Gynecologists stated ‘the available evidence suggests that magnesium sulphate given before anticipated early PTB reduces the risk of CP in surviving infants. Physicians electing to use magnesium sulphate for fetal neuroprotection should develop specific guidelines regarding inclusion criteria, treatment regimens, concurrent tocolysis, and monitoring in accordance with one of the larger trials’ [35]. Such guidelines would typically recommend inclusion at 24–31 completed weeks of gestation for both singletons and multiple pregnancies with pPROM, SPTL or elective PTB with expected delivery within 2–24 hours. A bolus of 5 g magnesium sulphate intravenously is followed by an infusion of 1 g/h in 24 hours or until delivery. Maternal adverse effects of magnesium sulphate like flushing, nausea, vomiting, hypotension and tachycardia are similar to those associated with the use of magnesium sulphate for the prevention of eclampsia.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


