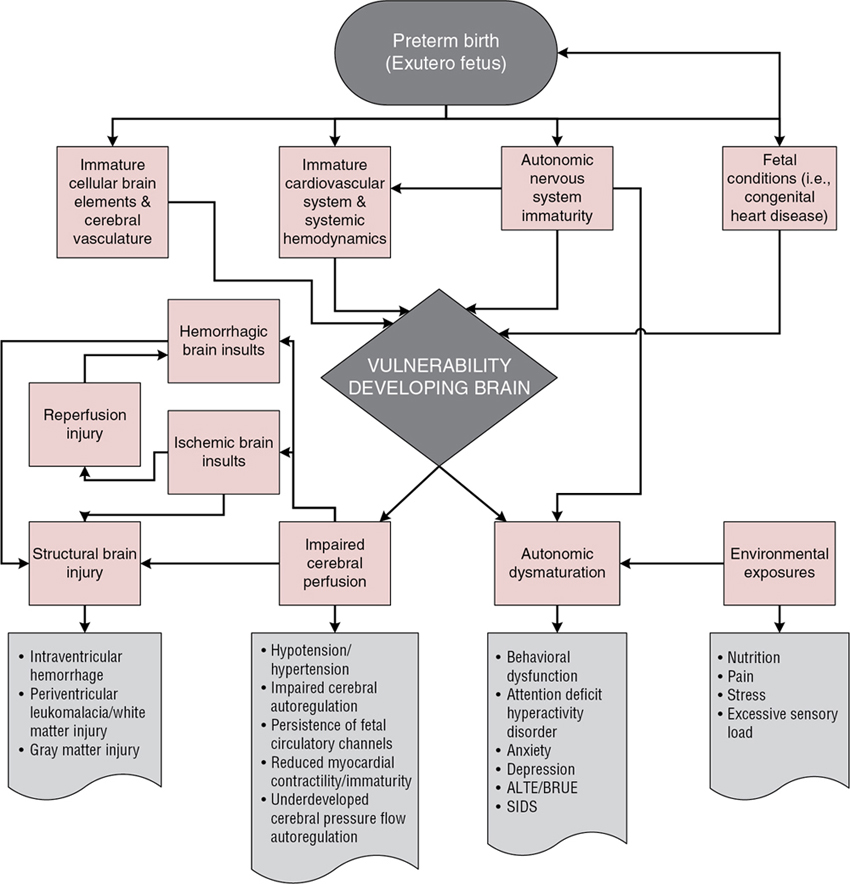
Sarah D. Schlatterer, Sarah B. Mulkey, Adre J. du Plessis Key points Regulation of brain perfusion and oxygen/substrate delivery is, broadly speaking, dependent upon two partially overlapping systems. These are cardiovascular-respiratory regulation by the brainstem, autonomic nervous system (ANS) centers, and intrinsic cerebral autoregulatory systems. The period of transition from the relatively protected intrauterine environment to the complexities of the external world requires the coordination of the newborn’s cardiovascular and respiratory systems. The ANS plays a central role in maintaining homeostasis for the infant under fluctuating external conditions. ANS maturation begins in the fetal period and continues after birth. Therefore intrauterine conditions that do not provide a supportive environment for ANS development, including certain maternal medical conditions or illnesses, placental insufficiency, and intrinsic fetal conditions (e.g., congenital heart disease [CHD]), and preterm birth, may significantly affect ANS development and function. ANS immaturity or abnormal maturation may leave vulnerable infants unprepared for ex utero respiratory and hemodynamic demands, exposing the infant to the risks of respiratory, cardiovascular, and hemodynamic instability. Importantly, immature ANS regulation and impaired hemodynamics may not only lead to brain injury but also result in a spectrum of more subtle neurodevelopmental changes whose effects may not become evident until later in childhood. Significant advances in obstetrical, neonatal, and cardiovascular intensive care, ventilation strategies, and management of neonatal hemodynamics have led to a decline in the earlier, often devastating, forms of brain injury seen in the preterm newborn, for example, cystic periventricular leukomalacia (PVL) and periventricular hemorrhagic infarction (grade IV intraventricular hemorrhage, [P/IVH]) and improved survival for other at-risk newborns, including those with CHD. Although survival of at-risk newborns has greatly improved and, in certain groups, there is a reduced prevalence of severe neuromotor disability and epilepsy among survivors, neuropsychologic disorders continue to manifest in ex-preterm children, former fetal growth-restricted children, and survivors of CHD. Thus neurologic morbidity remains prevalent in survivors of premature birth, high-risk near-term and term births, and cases of complicated fetal-neonatal transition. In this chapter we explore the immature ANS and its relation to brain injury and seek to understand the influence of the developing ANS on brainstem responses and higher cortical functions and outcome. There is a complex, potentially bidirectional relationship between immaturity and dysfunction at the brainstem level ANS centers and injury/developmental dysfunction in higher cerebral structures. The link between ANS maturation and brain injury has been most widely studied in the preterm population. Preterm infants with poor neurologic outcomes often have abnormal ANS maturation.1 ANS dysfunction and brain injury are also prevalent in other at-risk neonatal populations. For example, in infants with CHD, certain forms of which are known to cause delayed brain development, lower ANS function is associated with higher preoperative brain injury scores.2 In term neonates with neonatal encephalopathy from hypoxia-ischemia (hypoxic ischemic encephalopathy [HIE]) specific patterns of cortical injury were associated with ANS dysfunction.3 Similarly, neonatal stroke severity is inversely correlated with sympathetic tone.1,2,4 The causal pathway is not always clear in these cases, and further studies are needed to clarify these relationships. Whether ANS dysfunction causes brain injury or whether brain injury leads to ANS dysfunction remains debatable, and the interaction is likely reciprocal. The ANS regulates functions of the respiratory, cerebrovascular, and cardiovascular systems, and any abnormal function in these inter-related systems puts the immature brain at risk for injury or maldevelopment. Immature ANS responses likely contribute to hemodynamic and cardiovascular instability in at-risk infants, with impact on the cerebrovascular system.5,6 The prevailing paradigm for hemodynamically mediated brain injury in the premature infant is centered on a confluence of insults emanating from the unstable immature cardiovascular system and dysfunctional intrinsic cerebral autoregulation in the context of fragile cerebral vasculature. The brain’s cellular elements are also vulnerable to hypoxemia and inflammation, in particular, the immature oligodendrocytes, enhancing risk for brain injury from periods of hemodynamic instability. The ANS consists of the sympathetic and parasympathetic divisions and has integral control functions on many physiologic aspects of the human body. In addition, the ANS might also provide key inputs to the development of higher cortical and limbic structures involved in emotion, behavior, and thought processing. This important component of our nervous system matures during fetal development and into infancy.7,8 Key ANS centers within the brainstem, namely the nucleus tractus solitarius, receive sensory input from peripheral receptors and respond through ANS efferent systems, including the dorsal motor nucleus of the vagus. In addition, supratentorial ANS centers including the anterior thalamus, anterior cingulate gyrus, and the amygdala integrate the more primitive functions of the ANS with higher cortical processes, a major evolutionary advantage for humans. The influence of the impaired developmental ANS on these higher-order cortical structures may contribute to the high rate of psycho-affective disorders in survivors of an abnormal third-trimester environment in utero or ex utero.9 The central ANS develops in a “bottom-up” manner, beginning with brainstem and hypothalamic centers early in gestation. Cerebral ANS structures develop later in gestation and during early infancy. Functional maturation of the ANS begins with the sympathetic system, which develops structurally and functionally early in gestation and continues to develop progressively throughout the fetal period. While the unmyelinated vagal (parasympathetic) system is earliest to develop, it remains functionally quiescent until the third trimester when its function becomes integrated with higher cortical centers.10 The remainder of the parasympathetic system begins to develop after the sympathetic system and does not begin to exert functional influence until the third trimester, when parasympathetic tone increases significantly.5,10–13 Development in an unsupportive intrauterine or extrauterine environment (as may occur in preterm infants) can have significant effects on ANS function, resulting in alterations in hemodynamic control and risk for brain injury. Early delivery, even late preterm or early term, may be associated with ANS dysfunction.14 The premature engagement of the ANS in responding to postnatal cardiorespiratory changes may result in “dysmaturation”, or a shift in the temporal program of ANS maturation, and aberrant programming of the ANS.15 Prematurity and ANS development ex utero has been associated with impaired ANS function in several studies,16–19 and preterm infants with higher levels of prematurity-related complications appear to have more impaired autonomic function than preterm infants with low levels of complications.17–21 This association holds true for neurologic outcomes as well, as preterm infants with adverse neurologic outcomes have lower autonomic tone (measured by heart rate variability [HRV]) compared to age-matched infants with favorable neurologic outcomes.1 Measurement of ANS function is challenging in the fetus and fragile newborn. HRV, the fluctuation in the length of time between heart beats (R-R intervals), can be analyzed noninvasively to assess sympathetic and parasympathetic tone, providing a window on the developing ANS.7,22 High-frequency variability reflects parasympathetic function and is influenced by the respiratory rate (respiratory sinus arrhythmia), while low-frequency variability results from a combination of sympathetic and parasympathetic inputs and reflects baroreflex-induced changes in HR.23 HRV is also influenced by the newborns’ sleep state, with active sleep having higher sympathetic tone (low-frequency variability) compared to quiet sleep.7 For premature or critically ill term newborns, there are a multitude of “unexpected” stimuli in the ex utero environment, which the immature ANS may be unprepared to experience and process. The neonatal intensive care unit (NICU) or cardiovascular intensive care unit (CICU) environments are harsher than the muted intrauterine milieu for the developing neurosensory systems. Depending on the gestational age (GA) and morbidity of the infant, the extraordinary experiences may include oxygenation disturbances and positive pressure ventilation forces on the lungs, hemodynamic instability increased by a patent ductus arteriosus, infections, painful procedures, light, and air and temperature changes on the delicate skin, among others. These stimuli can create a challenging environment for maturation of the ANS, resulting in delayed or abnormal maturation. ANS dysmaturation may then increase the risk for certain adverse events, including IVH,24 sudden infant death syndrome, and brief resolved unexplained events (BRUEs, formerly “apparent life-threatening events” [ALTEs]),25–27 among others. In addition to prematurity, development in an unsupportive intrauterine environment, whether secondary to placental insufficiency or intrinsic fetal factors, may have consequences for ANS function. Growth-restricted fetuses (FGR) exhibit delayed/immature ANS function,28–30 as evidenced by depressed HRV28 and suppressed baroreflex and chemoreflex responses.19 Similarly, fetuses with specific types of critical CHD, including hypoplastic left heart syndrome (HLHS) and transposition of the great arteries (TGA), also have abnormal ANS function.11,31 Mechanisms underlying aberrant ANS development under the aforementioned conditions are not well understood. However, both FGR and critical CHD fetuses exhibit delayed brain growth and development.32–34 Poor growth and development of the fetus overall and of the central nervous system, specifically, likely contribute. The hemodynamic physiology of the developing brain increases its vulnerability to injury. Unlike the mature brain, where cerebral blood flow (CBF) exceeds the ischemic injury threshold by fivefold,35 the developing brain has significantly lower global and regional CBF,36 (see Chapter 2). These changes are most evident in the white matter, where there is a much lower ischemic injury threshold.37,38 This suggests a reduced margin of safety for cerebral perfusion but has to be considered in the context of reduced oxygen metabolism in the developing brain. Monitoring of cerebral tissue oxygenation during transition of the premature newborn during the first few hours after birth is an emerging and important approach to help protect the immature brain (Chapter 15).39 Under normal conditions, cerebral perfusion is maintained by a background perfusion pressure provided by the cardiovascular system, which is then “fine-tuned” within the cerebral vasculature by complex intrinsic autoregulatory mechanisms (Chapter 2). However, during sustained physiologic instability and after brain insults, these responses eventually fail, leading to brain injury.9,40 Cerebral pressure autoregulation maintains a relatively constant CBF across a range of cerebral perfusion pressures called the autoregulatory plateau.41 In addition to these upper and lower pressure bounds, cerebral pressure autoregulation also has a limited impulse-response time, of the order of 5–15 seconds.42 Outside these pressure and temporal bounds, CBF is pressure passive, with an increased risk of cerebrovascular injury. Cerebral pressure-flow autoregulation emerges during fetal life but is underdeveloped in the immature brain. With decreasing GA, the autoregulatory plateau is narrower and lower,43–45 and normal resting blood pressure (BP) is closer to the lower threshold of autoregulation.45 Although cerebral pressure-flow autoregulation is well characterized in children and adults, this is not the case for the newborn,45 least of all the sick premature infant.46–53 Some studies in stable preterm infants have suggested a lower autoregulatory limit of around 25–30 mmHg48,54,55; however, in the sick premature infant the existence and limits of cerebral pressure autoregulation remain controversial.46,47,52,56–60 The integrity of the fetal ANS is tested during the events of parturition. The sympathetic and parasympathetic nervous systems work to support and maintain the fetal HR and BP during periods of increased stress and uterine contractions, and then transition of the fetus at birth to extrauterine life. However, fetal-neonatal transition, especially for a fetus that has endured a suboptimal intrauterine environment for development of the ANS, or when preterm, presents a complicated and critical time for hemodynamic regulation. During most labors, fetal HR is monitored via Doppler ultrasound (US) cardiotocography (CTG), which is typically combined with a monitor for maternal HR and a tocogram to detect uterine contractions. CTG-measured fetal HR produces an average of fetal heartbeats and so does not provide the data of high enough temporal resolution necessary for quantitative fetal HRV analysis but can display changing HRV via the fluctuation in the fetal HR. Fetal ECG devices can provide a true measure of the R-R interval and allow for quantitative fetal HRV analysis, although they are not often used clinically at this time.61 The fetal HR is influenced by the current state of the ANS, both its maturational level and the intrinsic intrauterine environment. In a study of fetal lambs developing in a hypoxic environment over 12 days, ANS maturation was slower than that of normoxic fetuses over the same gestational interval.62 Thus chronic hypoxia may impair maturation of the fetal ANS and impact its function at the time of parturition. Fetal stress from acute hypoxia can manifest as a reduction in HRV and as a change in the baseline fetal HR. Fetuses may have a bradycardic or tachycardic response to hypoxia, depending upon the fetus’s compensatory mechanisms available and on the duration and level of the hypoxia-stress exposure.63 The Fetal Stress Index is measured by fetal HRV analysis and reflects parasympathetic tone, which correlates with arterial pH in a fetal lamb model.64,65 During parturition in low-risk human labors, there is a reduction in the parasympathetic tone, seen as lower fetal HRV.66 In this same study sympathetic tone in the fetal HR was higher during parturition than prior to the onset of labor, although the actual baseline fetal HR was not different between the pre-labor and labor periods.66 Fetal HRV is therefore dynamic and responds to chronic and acute hypoxia states and during parturition. Much has been learned regarding the effects of acute fetal hypoxemia in near-term or term labors from fetal lamb models. Fetal bradycardia can develop from the parasympathetic nervous system response to developing hypoxemia.63 The degree of fetal HR lowering relates to the severity and duration of the hypoxemic exposure in an effort for the fetus to lower myocardial metabolic demand and to increase stroke volume.63 Meanwhile, the fetus maintains BP by peripheral vasoconstriction, which is mediated by sympathetic activation. In a fetal lamb model exposed to repeated fetal umbilical cord occlusions causing fetal hypoxemia for 1 minute, every 5 minutes, the fetal lamb is able to maintain baseline fetal HR and BP between occlusions for an extended period of time.67,68 This experiment simulates the repetitive uterine contractions in a human labor and shows the importance of the ANS in supporting the fetal cardiovascular response. In the same experiment a shorter duration between umbilical cord occlusions (1-minute exposure every 2.5 minutes) resulted in the fetal lamb responding to the more significant fetal hypoxemia with an initial tachycardia, followed by bradycardia and falling of mean arterial pressure.67,68 The fetal lamb showed reduced cardiovascular compensation when exposed to more frequent hypoxemic events, with the ANS not able to adequately maintain the fetus in a normal range of HR and BP as time progressed. Another effect of shorter interval hypoxemia exposure is fetal tachycardia following the decelerations. This may be due to beta-adrenergic myocardial stimulation from adrenal activation to increase HR following the period of umbilical cord occlusion.69 Thus the fetal ANS helps to support the complicated process of parturition and, under most circumstances, to enable birth of an infant with normal cardiovascular function in the first minutes of postnatal life. Prematurity-related brain injury remains a major public health concern.70,71 Survivors of prematurity are at risk for long-term motor, cognitive, and psycho-affective disorders (Figure 8.1).72–74 Both the incidence and severity of brain injury in premature infants are inversely related to GA.74 Because advances in survival have been greatest among the sickest, smallest, most immature infants, that is, those at greatest risk for cerebral circulatory instability and brain injury,75 it is perhaps not surprising that survivors of extreme prematurity have the highest risk for the development of cerebral palsy.76 Of major concern are the 25 to 50% of ex-preterm children who demonstrate potentially debilitating behavioral or learning problems by school age,72,73,77,78 with moderate to severe impairment in academic achievement,79 and increased risk of autism spectrum disorder.74,80 The principal forms of prematurity-related brain injury are germinal matrix–intraventricular hemorrhage (GM-IVH) and injury to the parenchyma, particularly to the immature white matter. The germinal matrices in the periventricular regions of the developing brain are supported by a profuse but transient vascular system of fragile thin-walled vessels with a deficient basal lamina, no muscularis layer,81,82 and a predisposition to hypoxia-ischemia/reperfusion injury, increasing their vulnerability to rupture.83 These germinal matrices are also vulnerable to rupture during fluctuations in perfusion pressure, the so-called “water-hammer” effect.84 The most serious complications of GM-IVH are periventricular hemorrhagic infarction, with an adverse long-term outcome rate exceeding 85%,85,86 and post-hemorrhagic hydrocephalus,87 which has an adverse long-term outcome in up to 75% of patients.88,89 Fortunately, the incidence of this type of white matter injury has also declined.75 Under-vascularized end-zones in the premature cerebral white matter are susceptible to hypoxic-ischemic injury during periods of decreased perfusion pressure due to incomplete arterial in-growth during prematurity.90–96 White matter injury increases the risk of long-term neurologic impairment.97 The severe form of white matter injury, termed cystic PVL, that can be easily appreciated on US, now has a very low prevalence, but magnetic resonance imaging (MRI) studies98 describe a high prevalence of diffuse non-cystic white matter injury99,100 to which cranial US is insensitive.101 This diffuse form of white matter injury is detected by MRI in more than 50% of very premature infants.98,100 Cerebrovascular insults leading to both hemorrhagic and hypoxic-ischemic injury have long been considered a leading cause of acute and long-term neurologic morbidity in premature infants.102–106 Systemic hemodynamic impairment is commonly diagnosed and treated in the premature infant and is related inversely to GA at birth. This maturational association between disturbed systemic hemodynamics and cerebrovascular injury has led to the notion that the relationship is causative. However, despite plausible extrapolations from human adult and supporting animal studies,107–110 establishing a causal link between unstable hemodynamics and brain injury in the premature infant has many challenges. A variety of BP-associated disturbances have been implicated in prematurity-related brain injury, including arterial hypotension,111–118 hypertension,119,120 cerebral venous hypertension,121–123 and/or fluctuating BP.84,124,125 Different mechanisms have been proposed for the role of “hypotension” in GM-IVH. With intact pressure autoregulation, hypotension triggers cerebral vasodilation, with the increased cerebral blood volume potentially leading to the rupture of fragile vessels. With disrupted cerebral pressure autoregulation, or with BPs below the autoregulatory plateau, there is greater variability in CBF. During hypotension, hypoxic-ischemic injury to the vessel walls may further disrupt cerebral pressure autoregulation,126–133 and vessels may rupture during reperfusion. In addition to GM-IVH, systemic hypotension has also been implicated in the development of white matter injury in premature infants.111,112,134–136 However, other studies have shown no such relationship.116,120,137,138 The diastolic closing margin, the difference between the diastolic arterial BP and the critical closing pressure (the arterial BP at which CBF stops), is higher in premature infants that develop GM-IVH.139,140 However, critical closing pressure increases during the second and third trimesters, which may be why some premature newborns tolerate hypotension without resulting brain injury.141 Sustained hypertension is not commonly diagnosed in premature infants, although it may also result in the development of GM-IVH.119,120 Fluctuating BP in premature infants, particularly during positive pressure ventilation, has been associated with GM-IVH in a number of reports.51,112,114,119,124,125,142–144 However, the role of fluctuating cerebral perfusion in prematurity-related brain injury remains controversial, since several studies found no such association.47,125 The role of systemic BP disturbances and impaired cerebral pressure-flow autoregulation in prematurity-related brain injury is unclear, and further studies are needed to more appropriately address this important question. Doppler US and functional echocardiography studies have identified periods of low cardiac output and low “cerebral” perfusion in premature infants during the early hours after birth. These low-flow states are not reliably detected by systemic BP measurements145–149 and do not always respond to vasopressor-inotropes.148 These low cardiac output states are associated with low superior vena cava (SVC) flow, suggesting decreased cerebral perfusion.145,149–153 Periods of low SVC flow are associated with disturbed cerebral oxygenation by near-infrared spectroscopy (NIRS).154,155 Both the nadir and duration of low SVC flow are associated with severe GM-IVH,151,155,156 and later adverse neurologic outcome.145,149 The association between systemic BP disturbances, as currently measured and interpreted, and prematurity-related brain injury remains controversial (Chapter 7). Neonates with critical CHD are vulnerable to brain injury and are known to be at high risk for neurologic disability.157–161 There are multiple and cumulative influences beginning in the fetal period and extending through the postoperative phase that may contribute to these outcomes.162–165 Structural brain development and cerebral metabolism are impaired in critical CHD.159,160,166 White matter injury, including PVL, is commonly found both pre- and postoperatively.167,168 Preoperative brain injury in CHD appears to be strongly related to microstructural and metabolic brain development166 and may be related to differences in cerebral circulation.169 Fetuses and neonates may have impaired brain perfusion and oxygenation that likely contribute to delayed cerebral maturation and brain injury in this population.170,171 In the early transitional/neonatal period autonomic tone is depressed in infants with HLHS or TGA (both critical forms of CHD) when compared with healthy controls, supporting the notion that ANS development is delayed in the CHD population.11 Mechanisms underlying aberrant ANS function in neonatal CHD remain poorly understood. Delayed brain growth and development is known to occur in infants with critical CHD34,172,173 and may contribute to delayed development of the ANS. However, this remains to be proven. There appears to be a link between autonomic dysfunction and preoperative brain injury in neonates with CHD. HRV metrics correlate with preoperative brain injury scores in neonates with CHD, suggesting interplay between autonomic function and brain injury in this population.2 Depressed HRV precedes cardiac arrest in CHD infants,174 again supporting the role of the ANS in maintaining hemodynamic stability. Alterations in autonomic development in CHD may begin in the fetal period. Third-trimester fetal HRV is lower in fetuses with HLHS than in controls.31 A trend toward lower autonomic tone in fetuses with tetralogy of Fallot (ToF) and TGA has also been shown.31 Interestingly, fetal HRV correlates with 18-month developmental outcomes in survivors of CHD, supporting the association between ANS and neurocognitive function in this population.175 Several studies have found abnormal patterns of cerebral blood flow, including lower cerebrovascular resistance, in fetuses with CHD.176–178 This suggests that cerebral autoregulation in these fetuses is actively redirecting blood flow toward the brain. The timing of alterations in cerebral blood flow appears to correlate with the associated neurocognitive outcomes. Abnormal cerebro-placental ratios (CPR < 1) early in pregnancy (≤26 weeks’ GA), suggesting early cerebral hypoxia, correlate with lower 18-month cognitive development scores in CHD fetuses with HLHS, TGA, and ToF.179 However, lower cerebrovascular resistance later in pregnancy seems to correlate with better neurocognitive outcomes, indicating a need for more investigation into the role of cerebrovascular resistance in neurodevelopment in this population.180 Studies using frequency-domain near-infrared spectroscopy (FDNIRS) and diffuse correlation spectroscopy (DCS)181 and arterial spin-labeling techniques182 have shown that neonates with single-ventricle CHD have impaired CBF compared to controls. Votava-Smith et al. found fluctuating pressure-passive cerebral perfusion in full-term neonates with CHD,183 similar to what has been described in premature infants without CHD47 and in preterm infants with hemodynamically significant PDA.184 While several studies have shown decreased CBF in CHD, others have associated an increased CBF, in addition to other factors, including longer time to surgery and delayed sternal closure, with white matter injury in neonates with HLHS.185 Interestingly, fluctuating cerebral pressure-passivity can occur in the term preoperative infant with CHD despite systemic normotension.183 Cerebral pressure autoregulation also may be disturbed in the early postoperative period, particularly in the context of fluctuating systemic BP and higher CO2 tension.186 Several fundamental and inter-related challenges continue to impede our understanding of the relationship between changes in systemic hemodynamics, the immature response of the ANS, and brain injury in the developing brain. Advancing the field will require greater insight into the interaction between the immature systemic and cerebral hemodynamic systems, the ANS, and how this mediates injury in the immature brain. Currently, there are no established techniques for making continuous, quantitative measurements of systemic or CBF in the fragile newborn infant (Chapter 14). Arterial BP, the only continuous systemic hemodynamic signal available in the critically ill infant, has several limitations. First, there are no widely accepted noninvasive techniques for acquiring continuous BP in the critically ill infant.26,187–189 Second, the relationship between BP and systemic blood flow is not constant, particularly during periods of critical physiological instability. Arterial BP is often used as a surrogate for cerebral perfusion pressure because the effect of venous pressure is disregarded in many clinical situations. However, in critically ill infants requiring positive pressure ventilatory support, the effect of increased intra-thoracic pressure may have a significant impact on cerebral perfusion, and ventilator-related cerebral venous volume fluctuations have been shown to predispose to cerebral pressure passivity in a cohort that included preterm infants, infants with CHD, and term infants with HIE.190 At present, noninvasive techniques for monitoring cerebral perfusion pressure are not readily available for clinical use. There are currently no well-accepted techniques for the measurement of continuous volumetric CBF. Transcranial Doppler US measures CBF velocity,42,58,124,191–194 but not volumetric CBF. In the absence of reliable techniques for continuous CBF measurement, a number of intermittent (“static”) approaches have been used to measure quantitative CBF.57,145,149–151,195–199 These techniques are largely based on the Fick principle, using tracers ranging from Xe133 to oxyhemoglobin measured by NIRS. Another approach has been the use of intermittent measurement of SVC flow by Doppler US as a surrogate for CBF. These studies have demonstrated the association between abnormally low SVC flow and neurologic morbidity.145,149,150,152,153 However, these measurements of SVC flow are not continuous and, in the very preterm infant, less than 50% of the blood in the SVC represents the blood coming from the brain (Chapter 2).200 Thus all of the so-called “static” measurements of CBF suffer from the inability to capture the dynamic nature of cerebral hemodynamics during the period of major physiologic change associated with transition to premature postnatal life. The ability to measure the relationship between cerebral oxygen demand and supply would provide major insights into the mechanisms of brain injury in premature infants. This is important because it is clear that not only cerebral oxygen deficiency but also excessive cerebral oxygenation may be harmful to the immature brain.201,202 Even in stable infants, the premature brain might be “hyper-oxygenated” at room air compared with the mature brain.203 NIRS devices in current clinical use measure continuous cerebral tissue hemoglobin saturation as a surrogate measure of the adequacy of cerebral oxygen delivery (Chapter 15). The rationale behind this approach is that normal autoregulatory mechanisms maintain appropriate cerebral oxygen delivery and cerebral oxygen extraction9,140; conversely, increasing cerebral oxygen extraction is interpreted as a decrease in the cerebral oxygen delivery to demand ratio due to either decreasing CBF or increased cerebral activation. However, measures of cerebral tissue hemoglobin oxygenation may be misleading when considered in isolation because this approach assumes that neurovascular coupling is intact and because cerebral oxygen extraction may actually decrease after significant brain insults. Understanding CBF in the medically fragile infant has been limited by the lack of bedside techniques capable of measuring regional blood flow within the brain. Not only is global CBF lower in these infants, but blood flow is also particularly low in the most vulnerable white matter regions.204,205 Arterial spin labeling (ASL) perfusion MRI at 3 Tesla can measure regional cerebral perfusion noninvasively and without contrast.169,206 In a study using pseudo-continuous ASL, frontal lobe perfusion increased more rapidly with increasing GA compared to the occipital regions.204 In another study using ASL, neonates born prematurely had higher perfusion at term GA equivalence compared to term newborns, indicating a potential effect of ex utero development on brain perfusion.207 The ASL technique does have some technical challenges in the high-risk preterm neonate, and similar to many other imaging techniques, it is not portable to the bedside. Near-infrared spectroscopy in its various formats provides greater temporal resolution and is discussed in Chapter 15. Understanding the role of systemic hemodynamic factors in brain injury in medically fragile infants has been impeded by a fundamental lack of understanding of the “dose” of hemodynamic insult required to injure the immature brain. Insults capable of causing injury to the developing brain may be distinctly different from those injuring the mature brain. In the extremely premature infant the risk period for hypoxic-ischemic brain insults may extend for weeks or months during the prolonged NICU stay. In the infant with CHD exposure to (often multiple) bypass procedures and a persistently altered cardiovascular physiology results in prolonged, often life-long, risk for brain injury. Presumably, injury thresholds exist for brief but severe, mild but prolonged, and repetitive hemodynamic insults, as well as for the cumulative impact of these insults. It is difficult to design appropriate experimental models or to monitor the human premature brain due to the prolonged period of risk for brain injury and the variety of hemodynamic insults that may occur. Establishing a temporal association between systemic hemodynamic disturbances and brain insult is challenging in the medically fragile infant because the exact onset of brain injury is often unknown and brain injury may be cumulative.208 Furthermore, neurodevelopmental deficits may only become evident months to years after the initial injury, sometimes as late as school age,74,209 or beyond. During this interval, multiple factors, such as socioeconomic status and maternal education that are unrelated to the initial insult, may significantly influence long-term outcomes.210 In the premature infant brain injury precedes critical normal third-trimester events in brain development.211 Normal third-trimester developmental events may be derailed by acute injury resulting in acquired brain malformations (developmental disruptions).212 Conversely, the ameliorating effect of brain plasticity may play a beneficial role in outcome.213 Other potentially injurious mechanisms may operate before (e.g., fetal inflammation, altered fetal oxygen delivery), during (e.g., blood gas disturbances), and after (e.g., apnea and bradycardia, altered circulation, infection and inflammation214–216) the immediate period of postnatal hemodynamic instability and may act in concert with hemodynamic insults. Establishing a causal link between early hemodynamic insults and brain injury is also challenging; specifically, the identification of acute structural injury during periods of critical illness has been limited by the lack of sensitive portable neuroimaging. Infants are usually too ill to be transported to MRI scanners, especially early in their hospitalization. Cranial US, although sensitive to hemorrhage, is insensitive to and delayed in its detection of diffuse white matter injury. This has been confirmed at autopsy216,217 but also in MRI studies that indicate that most white matter injury is undetected by neonatal cranial US.98,218 MRI-compatible incubators allow for safer transport of critically ill infants to MRI scanners and may advance detection of hyperacute brain injury. Because the onset of cerebral pressure autoregulatory failure heralds an elevated risk for cerebrovascular injury at a point prior to irreversible injury, detection of cerebral pressure passivity might provide a sensitive cerebrovascular biomarker to relate to systemic BP changes. However, the presence and characteristics of pressure autoregulation in the sick premature infant remain controversial, in part due to the ongoing lack of established techniques for detecting cerebral pressure passivity. In a neonatal piglet model of hypotension cerebral autoregulation assessed by the linear correlation between arterial BP and rSO2 (measured by NIRS) was as accurate as cerebral autoregulation assessed by the linear correlation between invasively monitored cerebral perfusion pressure.219 The NIRS hemoglobin difference (HbD) signal, which can be measured continuously, can be time-locked to measurements of systemic BP and applied to the study of cerebral pressure-flow autoregulation in premature infants.46,186,220 This approach can identify periods of pressure passivity using coherence function analysis to identify significant concordance between systemic BP and cerebral HbD changes.186 The magnitude of pressure passivity during these periods can then be measured using transfer gain analysis.47 Using this approach, an association between cerebral pressure-passivity and brain injury has been described,46 as well as between the magnitude of pressure passivity and GM-IVH,220 and in the term newborn with HIE.221 This approach highlighted the prevalence and dynamic nature of cerebral pressure flow autoregulation, with periods of pressure passivity interspersed with apparent autoregulation in most extremely premature infants.47 Pressure autoregulation is affected not only by changes in arterial pressure but also by other influences, including reactivity to vasodilators such as carbon dioxide and the regulation of arterial pressure itself.47,222 Brain injury such as hemorrhage or stroke may affect cerebral autoregulation, as well as conditions that alter intracranial pressure (i.e., hydrocephalus).222 A continuous, robust cerebral circulation monitor that allows for both regional and global CBF assessment at the bedside is needed in this vulnerable population.222 Predictable gestational/postnatal age–related BP bounds remain difficult to determine and multiple, simultaneous influences are likely at play in the critically ill neonate. Because the ANS plays a key role in maintaining hemodynamic stability during transition and during critical periods for the fetus and vulnerable neonate, strategies to support ANS development and function are critical. To date, the focus has been on providing support in the ex utero environment, and studies have concentrated on the preterm infant who may need weeks to months of neonatal intensive care. Kangaroo care, the practice of placing the preterm infant skin-to-skin on the chest of its mother, has been studied in this context. Kangaroo care has been shown to support appropriate ANS development and neurobehavioral maturation (as measured by habituation and orientation scores on the Neonatal Behavioral Assessment Scale) in preterm neonates, as evidenced by increased sympathetic tone at 28 weeks’ GA223 and increased parasympathetic tone at 37 weeks’ GA.224 Kangaroo care combined with maternal singing had the added benefit of reducing maternal anxiety.225 Other simple interventions, such as pacifier use, may improve sympathetic tone in former preterm infants.226 The Family Nurture Intervention (FNI), developed by Welch and colleagues, also shows promise in supporting appropriate ANS development in preterm infants. Preterm infants who underwent FNI had more rapid increases in parasympathetic function in HR data collected between ∼35 weeks’ and ∼40 weeks’ GA compared with standard-of-care controls, although there was no group difference in HR at term age.227 In a follow-up study of the same cohort at 5 years, children and mothers who participated in the FNI had higher vagal tone than the standard-of-care control group.228 Even when an infant is exposed to non-optimal conditions during critical periods of development (i.e., prematurity), non-pharmacologic interventions including skin-to-skin contact, pacifier use, and FNI may promote healthier ANS development. Maternal stress and mood disorders are some of the most common complications of pregnancy,229–231 and studies have shown an association between maternal depression, stress, and delayed ANS development during the fetal period,232–235 although these are not always straightforward. An association between maternal and fetal HR and infant HRV at 1 year of age has been reported by DiPietro and colleagues.236 Lower parasympathetic tone was found in infants of mothers with depressive symptoms in the third trimester237 and in infants of mothers with high anxiety in the second trimester.238 Conversely, other studies found no correlation between maternal negative trait emotionality and infant parasympathetic tone in pregnant adolescents, suggesting that the effect of maternal depression and anxiety during pregnancy on the brainstem ANS may be transient.239,240 The type and degree of maternal stress or mental distress experienced as well as underlying genetic and epigenetic factors may impact the influence of maternal mental state on fetal and neonatal autonomic function. There is a need for future studies to better understand relationships between maternal mental health and the developing ANS. Successful transition of the fetus to the extrauterine environment is mediated by the most complex physiological changes anywhere across the lifespan. For premature and critically ill newborns, transition may be suboptimal due to, among others, immaturity of the systems necessary for the effective regulation of HR, BP, and CBF. Brain injury may result due to impaired regulation of these systems and may translate into a long-term neurologic burden of neurodevelopmental delay, cerebral palsy, and psycho-affective disorders that may persist across the lifespan. In this chapter we have outlined evidence for the key role the ANS plays in hemodynamic stability and neuroprotection and have explored the fundamental challenges in our understanding of systemic and cerebral hemodynamics and the immature ANS, as well as the limitations of current techniques for addressing these complex but critically important questions. The etiology of brain injury in premature and at-risk term newborns is multifactorial and the insult may be chronic and cumulative (Figure 8.1). The ANS is an integral driver in brainstem control of HR and BP, but more importantly, at least for the long-term neurologic health of infants, it is emerging as an important brain system for promoting affect, mood, stress responses, and behavior through its linkages to higher order cortical centers.62,200 Much remains to be learned, and in order to support ANS development and maturation in at-risk neonates, further understanding of ANS development and factors that support or impede that maturation is critical. Innovative techniques and novel tools for monitoring and measuring autonomic function will be required to better understand the interplay between the ANS and its higher cortical and subcortical systems as they relate to brain injury and long-term neurodevelopmental and neuropsychological dysfunction in vulnerable populations.
Chapter 8: The immature autonomic nervous system, hemodynamic regulation, and brain injury in the early developing brain
Introduction
Why focus on the developing ANS?
ANS development and metrics
Cerebral hemodynamic control in the developing brain
Autonomic function and hemodynamics in labor
Prematurity, brain injury, and systemic hemodynamic disturbances
Congenital heart disease, autonomic function, and cerebral hemodynamics
Resolving the relationship between systemic hemodynamics and brain injury: Obstacles to progress
Measurement of relevant hemodynamic and metabolic indices
Characterizing “significant” systemic hemodynamic insults and establishing a temporal relationship between hemodynamic changes and brain insults difficult in neonates
Future directions
Conclusion
References