9 The Evidence Base
Definition of Evidence-Based Practice
The term evidence-based practice was coined in the 1990s and has been widely embraced by most healthcare disciplines.1 Evidence-based practice (EBP) integrates the best scientific research evidence with clinical expertise of healthcare practitioners and patient and/or family preferences to facilitate clinical decision making.2 Decisions are based upon factors from each domain: the clinical situation, the patient and family preferences, and the research evidence, and individualized to the circumstances. There are multiple models of EBP between and within healthcare disciplines. The conceptual model described by Satterfield et al is a interdisciplinary model based upon the strengths of EBP models and processes from medicine, nursing, psychology, social work, and public health1 (Fig. 9-1). The strength of pediatric palliative care practice stems from the interdisciplinary collaboration of multiple healthcare disciplines. EBP is an approach to problem solving that addresses the needs of the organization, the individual practitioner, the patient, and the family to promote high-quality healthcare. Organizational experiences of quality improvement and financial data, combined with clinical research, clinical experience, and patient and/or family preferences promote shared decision making based upon all contextual factors.3 Palliative care is a relatively young discipline and pediatric palliative care is even younger. The evidence base in palliative care is not robust and there is a paucity of evidence specific to pediatrics.4
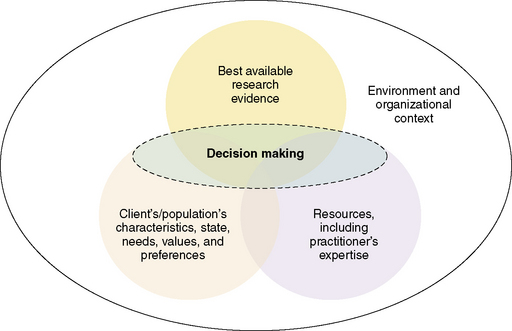
Fig. 9-1 The interdisciplinary model of evidence-based clinical decisions.
(Adapted from Satterfield et al. Toward a transdisciplinary model of evidence based practice. Milbank Quarterly, 2009; 87(2), 368–390.)
Evidence-based practice is a process as well as a practice descriptor.5 The process involves defining a practice issue, searching the literature for current research, critically appraising the evidence, synthesizing the evidence to make recommendations for changes in practice, implementing the recommended changes, and evaluating the outcomes. EBP relies on a holistic approach to decision making based less upon expert opinion. Translation of the domains of EBP, scientific research, clinical expertise, and patient and/or family preferences into practice produces products such as changes in clinical practice, clinical guidelines, organizational standards of practice, and opportunities for fostering a research agenda in pediatric palliative care.
The healthcare professions involved in pediatric palliative care, including medicine, nursing, psychology, social work, child life and others, are a science and applied professions with equal contributions to the art and science of clinical practice. The perceived value of evidence may vary depending upon the discipline of practice. Traditional evidence-based medicine relies heavily on randomized clinical trials, yet this approach to generations of evidence is not as practical for pediatric palliative care, where a vulnerable patient population is often excluded from clinical research.6 Most palliative care research is adult-focused and this is one of the challenges to creating pediatric palliative care evidence-based guidelines (Table 9-1). The narrow scope of pediatric palliative care may be too focused for most standard literature search engines. The value of expert clinicians within and between disciplines is unclear and often underestimated in determining EBP recommendations. Evidence must be defined broadly and considered within the context of the specific clinical issue. Evidence is published in a wide variety of journals pertinent to a spectrum of diseases.
TABLE 9-1 Assumptions and Challenges of Evidence-Based Practice in Pediatric Palliative Care
* (Pediatrics is defined as the care of children, adolescents and young adults.)
Adapted from Satterfield, JM, Spring, B, Brownson, RC, Mullen, EJ, Newhouse, RP, Walker, BB, et al. (2009). Toward a transdisciplinary model of evidence-based practice. The Milbank Quarterly, 87 (2), 368-390; and Newhouse, RP, Dearholt, SL, Poe, SS, Pugh, LC, & White, KM (2007). Johns Hopkins Nursing Evidence-Based Practice Model and Guidelines. Indianapolis: Sigma Theta Tau International.
Levels of Evidence Used in Practice Guideline Development and Implications for Pediatrics
The development of clinical practice guidelines for the management of a variety of diseases and conditions brings together experts to review and summarize current evidence, evaluate the quality of the evidence, and make recommendations for practice. The National Consensus Project for Quality Palliative Care (NCP) brought together leaders of five national organizations representing hospice and palliative care. This task force followed a rigorous process of evidence review and consensus development to produce the Clinical Practice Guidelines for Quality Palliative Care in 2003, with an update in 2009.7 The National Quality Forum, a private, nonprofit membership organization recognized for its national leadership in healthcare quality improvement, reviewed and adopted the NCP guidelines as the basis for its Framework for Palliative and Hospice Care Quality Measurement and Reporting. Clinicians and clinical organizations identified 38 best practices for implementation.8 Quality indicators are being developed to evaluate the impact of those practices.
All clinical recommendations are not equivalent. Their importance will vary with the likelihood of significant benefit or harm avoidance to the patient. The evidence and experience that provides the foundation for the recommendation will also vary in quality depending on the methods used to produce the results. Frameworks have been developed to rate these factors within a set of clinical guidelines. These frameworks use a letter designation for how strongly the practice recommendation is made or how uniform the consensus is for the recommendation, and a numeral designation for the quality of the evidence supporting that recommendation (Tables 9-2 and 9-3).
TABLE 9-2 Framework for Rating Recommendations for Clinical Practice
| Strength of recommendation | Quality of evidence for the recommendation | Modified coding for pediatrics |
|---|---|---|
| I: One or more randomized controlled trials with clinical outcomes and/or validated laboratory endpoints | I: One or more randomized trials in children* with clinical outcomes and/or validated laboratory endpoints | |
| I*: One or more randomized trials in adults with clinical outcomes and/or validated laboratory endpoints with accompanying data in children* from one or more well-designed, nonrandomized trials or observational cohort studies with long-term clinical outcomes | ||
| II: One or more well-designed, nonrandomized trials or observational cohort studies with long-term clinical outcomes | II: One or more well-designed, nonrandomized trials or observational cohort studies in children* with long-term clinical outcomes | |
| II*: One or more well-designed, nonrandomized trials or observational cohort studies in adults with long-term clinical outcomes with accompanying data in children* from one or more smaller nonrandomized trials or cohort studies with clinical outcome data | ||
| III: Expert opinion | III: Expert opinion |
* Studies that include children or children and adolescents but not studies limited to post-pubertal adolescents.
Adapted from Panel on Antiretroviral Guidelines for Adults and Adolescents: Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents; Guidelines for the Use of Antiretroviral agents in Pediatric HIV Infection. http://aidsinfo.nih.gov/Guidelines/GuidelineDetail.aspx?MenuItem=Guidelines&Search=Off& GuidelineID=7&ClassID=1, and http://aidsinfo.nih.gov/Guidelines/GuidelineDetail.aspx?MenuItem=Guidelines&Search=Off&GuidelineID=8&ClassID=1. Department of Health and Human Services. Published August 16, 2010.
TABLE 9-3 National Comprehensive Cancer Network Categories of Evidence and Consensus
| Category 1: The recommendation is based on high level evidence (e.g., randomized controlled trials) and there is uniform NCCN consensus. |
| Category 2A: The recommendation is based on lower level evidence and there is uniform NCCN consensus. |
| Category 2B: The recommendation is based on lower level evidence and there is non-uniform NCCN consensus (but no major disagreement). |
| Category 3: The recommendation is based on any level evidence but reflects major disagreement. |
| All recommendations are category 2A unless otherwise specified. |
National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. NCCN categories of evidence and consensus. Available at http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
Other research designs that generate evidence include prospective non-randomized trials and observational cohorts; retrospective record reviews, interviews, or surveys; cross sectional studies; qualitative, ethnographic research; and case series or reports. Observational studies may yield associations but cannot determine cause and effect; hence, they are rated lower than RCTs for quality of evidence to support a particular clinical intervention. Similarly, qualitative studies are considered a source of credible evidence but are considered less informative about cause and effects than RCTs.9 Expert consensus is the least-rigorous level of evidence but recognizes the knowledge accumulated through serial observations by experts in the field. It is therefore possible to have strong recommendations that are supported only by expert opinion.
The Working Group on Antiretroviral Therapy and Medical Management of HIV-Infected Children is made up of experts from different disciplines. This group is rating their clinical recommendations in the manner described above. However, they recognized that some of their strongest recommendations were based on the adoption of practices proved efficacious in rigorous, well-powered clinical trials of adult patients with minimal supporting evidence in a pediatric population. Following the framework above would result in a rating the supporting evidence as Level III—Expert Opinion. Yet this did not adequately reflect the high quality and compelling results of the evidence that could and should be applied to children with the same life-threatening condition. Therefore the Working Group has modified its rating system to recognize the application in pediatrics of evidence developed in studies of adult patients. An asterisk is added to the numeral to designate that the primary evidence is taken from adult studies and that there is evidence in smaller and more focused pediatric studies that support its application in children.10
Ideally, strong evidence underpins all treatment decisions, yet many clinical situations still demand that clinicians use their best professional judgment in the absence of conclusive evidence. This is precisely the definition of evidence-based practice: the integration of individual clinical expertise with the best available external clinical evidence in the care of individual patients.11
There are several reasons why a conclusive level of evidence is difficult to obtain in pediatric palliative care. First, designing and conducting randomized trials with seriously ill or dying patients of any age requires meticulous attention to the treatment alternatives, the burden of outcome assessment,12 and the need for short-term outcome measures given the likelihood that patients may die early in the study. Second, many important questions in palliative care are not answerable through randomized clinical trial design. Finally, the questions that can be answered through RCT, such as comparing two medications for treatment of a specific symptom, are more difficult to conduct in pediatric populations due to a smaller sample of eligible patients, the wide variation in physical development associated with age, and the diversity of disease conditions.13 Although not ideal, pediatric clinicians must sometimes make treatment decisions based on strong evidence obtained in adult trials and the presumption that there is likelihood of similar benefit in children.14



