Fig. 4.1
A single, calcified metastatic osteosarcoma nodule in the left lung (identified with arrow)
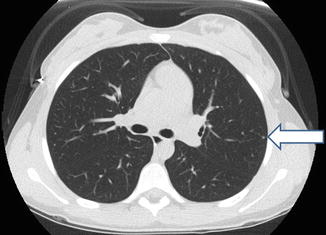
Fig. 4.2
A single, biopsy-proven benign left lung nodule (identified with arrow)
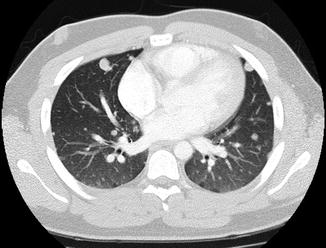
Fig. 4.3
Multiple metastatic Ewing nodules
4.2.2 FDG-PET
18F-fluorodeoxyglucose positron emission tomography (FDG-PET) is increasingly being used as a staging tool for patients with cancer, including malignant bone tumors. Unfortunately, while traditional FDG-PET is very useful in the evaluation of chemotherapeutic response and is an excellent screening tool for lymph node and bone metastases, its utility in the evaluation of lung nodules is limited. A standardized uptake value (SUV) of 2.5 has been used as a cut off to distinguish benign from malignant lesions (Cistaro et al. 2012). Multiple studies have demonstrated the sensitivity of FDG-PET in the identification of malignant lung nodules to be inferior to that of CT, especially for smaller lesions (Franzius et al. 2001; Volker et al. 2007; Fortes et al. 2008). It is particularly unreliable for nodules less than 8–10 mm in size, with reported sensitivity rates as low as 25–29.6 % (Absalon et al. 2008; Brader et al. 2011). The addition of computed tomography to FDG-PET (FDG-PET/CT) has allowed improved accuracy in the evaluation of lung nodules with this modality. Sensitivity and specificity rates of 90.3 % and 87.5 %, respectively, have been reported with PET/CT, but unfortunately, this modality still demonstrates limitations in the evaluation of tiny nodules less than 6 mm in size (Cistaro et al. 2012).
4.3 Management of Lung Nodules
4.3.1 Management of Lung Nodules in Osteosarcoma Patients
As previously noted, about 20 % of patients will present with identifiable metastatic disease at diagnosis, with pulmonary disease being present in over 80 % of these cases (Kempf-Bielack et al. 2005). Without systemic therapy, more than 80 % of patients will develop lung recurrence regardless of local surgery (Link et al. 1986). Unfortunately, patients with metastatic disease have a dismal outcome overall, with long term survival rates of less than 20 % (Mialou et al. 2005). Several factors have been identified that influence outcomes in OS patients with lung disease, including the total number of nodules, unilateral or bilateral involvement, and the ability to achieve a complete surgical resection. Presenting with more than three to five pulmonary nodules has consistently been identified in several studies as being associated with inferior survival (Buddingh et al. 2010; Chen et al. 2008; Bacci et al. 2008; Daw et al. 2006). For those with three or fewer nodules, 5 year overall survival rates of 40 % or greater have been described in some analyses, as compared to less than 15 % for those with higher numbers of nodules. Likewise, bilateral involvement has been shown to confer a worse prognosis for OS patients, with 5 year disease-free survival rates of less than 10 % having been reported for those with bilateral lung metastases at presentation (Bacci et al. 2008). It has also been reported that patients who present with centrally located nodules (defined as lesions adjacent to a first degree bronchus or blood vessel) have an inferior prognosis compared to those with peripheral lesions (Letourneau et al. 2011b).
The therapeutic intervention that has been shown to have the most significant impact on survival for patients with pulmonary metastases is the ability to achieve a surgical complete remission. While chemotherapy is necessary for any attempt at cure for patients with OS, without resection of all lung disease long-term disease survival is nearly impossible (Bacci et al. 2008; Buddingh et al. 2010; Kager et al. 2003). The accepted surgical approach to lung nodule resection is via thoracotomy, which allows for direct palpation of lung tissue to identify lesions not identified on pre-operative imaging. Despite advancements in imaging technology, studies continue to demonstrate that CT often underestimates the number of nodules found when thoracotomy is performed (Kayton et al. 2006; Cerfolio et al. 2009). Thus, while thorascopic approaches to nodule resection may have less morbidity, the importance of complete surgical resection of all metastatic disease continues to support thoracotomy as the standard for the management of OS patients (Castagnetti et al. 2004). Thoracoscopy may have a role when the diagnosis of metastatic disease is in question and the nodules are large enough to be amenable to this approach. The role of bilateral thoracotomy in the management of patients who present with unilateral disease continues to be an area of debate. Data on the optimal management of patients with unilateral disease at presentation is lacking, but a report published by Su et al. supported the performance of a contralateral thoracotomy for patients who recurred with unilateral disease less than 2 years from diagnosis. In this study, seven of nine (78 %) of patients with recurrence <2 years from diagnosis went on to develop contralateral disease, while only one of five (20 %) of patients who developed unilateral disease more than 2 years from diagnosis went on to develop contralateral recurrence. A survey of surgeons and oncologists performed by the Connective Tissue Oncology Society revealed that thoracotomy of the unaffected side has not been routine practice of most surgeons and oncologists, and at least one subsequent retrospective analysis showed no advantage to contralateral surgery (Su et al. 2004; Vo 2014). Further prospective studies are necessary to develop firm recommendations on this topic, though such trials are unlikely to be undertaken due to the invasive nature of the intervention.
4.3.2 Management of Lung Nodules in Ewing Sarcoma Patients
Approximately 25 % of patients with EWS present with metastatic disease at diagnosis and similar to OS patients, hematogenous spread into the lungs is the most common site of distant disease. Despite improvements in survival with intensification of systemic chemotherapy for localized disease, outcomes remain poor for patients with metastatic EWS, with event-free survival consistently shown to be about 20 % (Pinkerton et al. 2001; Miser et al. 2004). Patients with metastatic disease exclusive to the lungs, however, have demonstrated better outcomes with disease-free survival of 30–40 % when treated with standard therapy (Pinkerton et al. 2001; Miser et al. 2004). Further, intensification of systemic therapy for patients with primary lung metastasis, utilizing high dose chemotherapy/stem cell rescue has been advocated by some, but the results of prospective studies have been mixed, and at this time, this approach should be considered investigational (Meyers et al. 2001; Drabko et al. 2012; Oberlin et al. 2006).
Local therapy is an important component of any treatment strategy aimed at prolonging survival in patients with metastatic EWS (Thorpe et al. 2012). While there have been no prospective studies designed to elucidate the optimal approach to local therapy, both surgery and radiation therapy can be considered, and may have a role in the management of pulmonary metastases. Several retrospective analyses have suggested benefit to the use of whole lung radiotherapy at doses of 12–18 Gy, and it has become standard practice in most centers (Paulussen et al. 1998a, b; Paulino et al. 2013; Spunt et al. 2001). The risk of radiation pneumonitis at these doses is relatively low, and severe pulmonary complications are seen in less than 10 % of those treated (Bolling et al. 2008). Unfortunately, the risk of toxicity does increase with subsequent surgical procedures or further radiation boosts to previously treated lung tissue (Bolling et al. 2008). The role of surgical resection is less clear in EWS patients with lung disease compared to OS patients. Two small retrospective analyses have suggested a survival benefit to surgical resection of EWS lung nodules (Lanza et al. 1987; Letourneau et al. 2011a). In an analysis performed by Letourneau et al. there was a suggestion that surgical resection of pulmonary nodules was associated with increased survival compared to patients treated with radiation, either alone or in conjunction with surgery (Letourneau et al. 2011a). The patient numbers in this analysis were quite small however, and the retrospective nature of the study prevents strong conclusions from being made. Further study is necessary to determine the optimal approach to local therapy for these patients.
4.4 Indications for Biopsy
Because pulmonary metastatic disease has such a significant impact on the prognosis and management of patients with bone cancer, attempts should be made to determine the etiology of lung nodules when possible. In many cases, the presence of lung disease is clear: large nodules greater than 5 mm in size involving both lungs, calcified in patients with OS, or nodules large enough to be positive on PET, likely need no further work-up at diagnosis to plan treatment. In cases where the diagnosis may be in question, the nodules will generally be amenable to video assisted thorascopic surgery (VATS) or CT guided biopsy. These techniques can establish a tissue diagnosis with minimal healing time, allowing for a more rapid initiation of chemotherapy. For small nodules, VATS following the pre-operative placement of microcoils or guidewires via CT guidance can be an effective way to biopsy lesions smaller than 5 mm in size (Fig. 4.4) (Heran et al. 2011; Murrell et al. 2011). Alternatively, lesions smaller than 5 mm in size can be observed following treatment with chemotherapy. Should the nodules increase in size with treatment, biopsy should be performed. In patients with osteosarcoma, complete resolution of nodules with chemotherapy occurs less than 10 % of the time (Bacci et al. 2008). Most nodules will remain stable, and potentially become more calcified. Resolution of pulmonary disease is much more likely for patients with Ewing’s sarcoma with resolution following induction chemotherapy seen in almost 50 % of patients (Luksch et al. 2012). A lack of complete resolution in this cohort was associated with a much worse prognosis. While a good response to chemotherapy in patients with EWS supports a diagnosis of metastatic disease, it may be difficult to completely exclude the possibility of other etiologies, such as infection or atelectasis, with resolution. Because most of these patients will likely receive whole lung irradiation which carries potential for morbidities, it is important to try to characterize the lesions at diagnosis if at all possible.
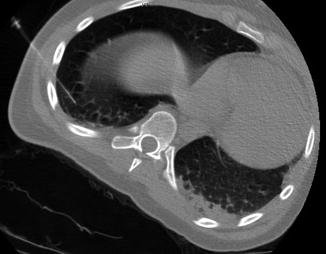

Fig. 4.4
CT guided localization of a tiny osteosarcoma lung nodule



