aG1, G2, or G3.
bEndocervical glandular involvement only should be considered as stage I and no longer as stage II.
cPositive cytology has to be reported separately without changing the stage.
Source: From Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103–104, with permission.
EPIDEMIOLOGY AND RISK FACTORS
KEY POINTS
- Type I cancers are estrogen related, and type II cancers are not.
- Type II cancers represent the more aggressive phenotypes.
- There is a high risk of an invasive cancer in the presence of biopsy-proven complex atypical hyperplasia (CAH).
Two broad histologic categories of endometrial cancer have been described (Table 8.2), termed type I and II carcinomas. They appear to have different patterns of molecular alterations that underlie their pathogenesis and clinical outcomes.
Table 8.2 Comparison between Type I and Type II Endometrial Cancers
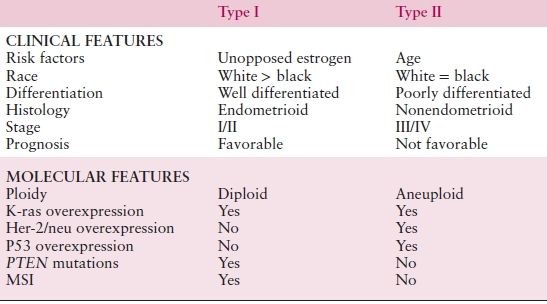
Type I endometrial cancers are associated with unopposed estrogen exposure and are often preceded by premalignant disease. They are usually early stage at diagnosis, are low-grade tumors (predominantly of endometrioid histology), and carry a favorable prognosis. Obesity and family history remain two of the strongest risk factors for endometrial cancer. Hereditary cancers are most clearly linked to Lynch syndrome (hereditary nonpolyposis colorectal cancer [HNPCC]), an autosomal dominant cancer susceptibility syndrome. Diabetes and hypertension, once thought to be risk factors for endometrial cancer, are probably surrogates for obesity; as a result, they probably should not be considered as important independent risk factors. Finally, tamoxifen (a common agent for the treatment of breast cancer) is a risk factor for the development of type I endometrial carcinoma, though the benefits of treatment (for breast cancer) far outweigh the risk of disease.
In contrast, type II endometrial cancers represent estrogen-independent tumors and are associated with a more aggressive clinical course. Unlike type I tumors, there is no readily observed premalignant phase. Both clear cell and serous carcinoma and perhaps grade 3 tumors comprise these histologic phenotypes. Some experts also classify uterine carcinosarcomas as a representative of type II histology (Table 8.2).
The molecular defects associated with endometrial cancers are stratified between type I and type II cancers. Type I cancers frequently have PTEN, KRAS, and PIK3CA mutations, and this is also varied by race, whereas type II cancers are more likely to have TP53 mutations. Among women belonging to families with the autosomal dominant HNPCC syndrome, the most common extracolonic malignancy is endometrial carcinoma with a lifetime risk of 40% to 60%.
PATHOLOGY OF PREINVASIVE DISEASE
The current classification of endometrial hyperplasia accepted by both the International Society of Gynecologic Pathologists (ISGP) and the World Health Organization (WHO) is based on the schema of Kurman et al., which divides hyperplasia on the basis of architectural features into simple or complex and on the basis of cytologic features into typical or atypical. The resulting classification relies on a combination of architectural and cytologic criteria. It comprises four categories as follows: simple hyperplasia (SH), complex hyperplasia (CH), simple atypical hyperplasia (SAH), and CAH. Endometrial hyperplasia appears to arise as a result of unopposed and prolonged estrogen stimulation. Therefore, some hyperplasias may regress if the estrogenic stimulus is removed.
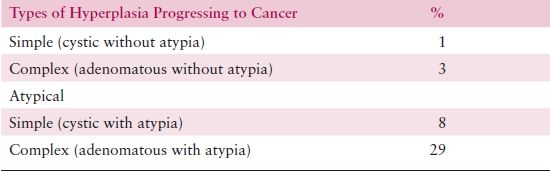
The probability of progression to adenocarcinoma is related to the degree of architectural or cytologic atypia. While progression occurs rarely for patients with SH or CH (incidence is 1% and 3%, respectively), progression from atypical hyperplasia is much higher: 8% and 29% for patients with SAH or CAH, respectively (Table 8.3). However, coexistent adenocarcinoma may be present in up to 40% of hysterectomies performed to treat CAH.
Table 8.3 Classification of Endometrial Hyperplasia
Mutter et al. have proposed an alternative classification of hyperplasia based on a combination of morphologic, molecular, and morphometric information. This includes the use of a new term, endometrial intraepithelial neoplasia (EIN), as the histopathologic presentation of a monoclonal endometrial preinvasive glandular proliferation that is considered the immediate pathologic precursor of endometrioid endometrial adenocarcinoma. Almost 40% of women with an EIN diagnosis will be diagnosed with endometrial carcinoma within 1 year, and for those who do not develop cancer within 12 months, there is a 45-fold increased risk of future endometrial cancer. Correspondingly, absence of an EIN lesion in an initial representative biopsy, including those with only benign hyperplasia, confers very high (99%) negative predictive value for concomitant or future adenocarcinoma.
In contrast, endometrial intraepithelial carcinoma (EIC) has recently been recognized as a histologically distinctive lesion that is proposed to represent a form of intraepithelial tumor. It appears to be the likely precursor to serous carcinoma of the endometrium.
PATHOLOGY OF ENDOMETRIAL CANCER
Uterine tumors are classified based on the ISGP and WHO classification system, which is a relatively simple classification scheme and accommodates the vast majority of endometrial carcinoma. It has been shown to accurately distinguish prognostic classes of endometrial neoplasms.
The differentiation of a carcinoma is expressed as its grade. Grade 1 lesions are well differentiated and are generally associated with a good prognosis. Both architectural criteria and nuclear grade are used to determine grade. The architectural grade is determined based upon the amount of the tumor growth in solid sheets (Table 8.4). The FIGO rules for grading state that notable nuclear atypia, inappropriate for architectural grade, raises the grade of a grade 1 or grade 2 tumor by 1. Though FIGO did not define notable nuclear atypia, it has been interpreted to include tumors with a majority of cells having nuclei of grade 3, which portends a significantly worse behavior and justifies upgrading by one grade. Some cell types (i.e., serous and clear cell) are not easily architecturally graded because their growth patterns are architecturally limited, and in these cases the nuclear grading is more universally applicable. In instances where two distinctive cell types are identified within a single tumor (mixed carcinomas), classification is based on the component that constitutes at least 10% of the tumor.
Table 8.4 Histologic Grade and Depth of Invasion
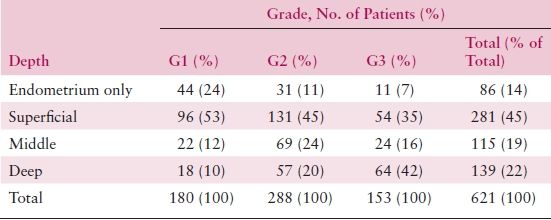
Source: Reprinted from Creasman WT, Morrow CP, Bundy BN, et al. Surgical pathologic spread patterns of endometrial cancer: A Gynecologic Oncology Group study. Cancer. 1987;60:2035–2041, with permission.
Molecular Alterations in the Pathogenesis and Progression of Endometrial Adenocarcinoma
Deletions or mutations of the PTEN gene and microsatellite instability (MSI) due to hypermethylation of the promoter for the mismatch repair gene, hMLH1, are both relatively common and early events in the development of a significant proportion of endometrioid adenocarcinomas. In contrast, these molecular alterations do not appear critical in the pathogenesis of serous or clear cell carcinoma. There are relatively few data to inform the importance of MSI as a prognostic factor in endometrial cancer, although defects in mismatch repair systems may alter responsiveness to chemotherapy or radiation.
Mutations in the p53 gene are found with high frequency not only in invasive serous carcinoma but also in EIC, the noninvasive precursor of serous carcinoma, suggesting that a different pathway is followed in the development of this second type of endometrial adenocarcinoma. These mutations appear to be an early event in the development of serous carcinoma, but a late event in endometrioid carcinomas for which it serves as an indicator of poor prognosis. In addition to the very frequent overexpression of p53 protein in serous carcinoma, it has also been related to high FIGO stage, clear cell histology, higher histologic grade, and increasing depth of myometrial invasion.
The KRAS, CTNNB1 (β-catenin), PIK3CA, and FGFR2 genes are also commonly mutated in endometrial cancer.
Cell Types
Endometrioid
Endometrioid adenocarcinoma is the most common form of carcinoma of the endometrium, comprising 75% to 80% of the cases. It varies from well differentiated to undifferentiated. Characteristically, the glands of endometrioid adenocarcinoma are formed of tall columnar cells that share a common apical border. With decreasing differentiation, there is a preponderance of solid growth rather than gland formation.
Foci of squamous differentiation are found in about 25% of endometrial adenocarcinomas. In a Gynecologic Oncology Group (GOG) study of early-stage disease, it was noted that these tumors with squamous regions behave in a fashion similar to endometrioid carcinomas without squamous differentiation. Historically, the tumors were sometimes separated into adenoacanthoma or adenosquamous carcinoma based on whether the squamous component appeared histologically benign or malignant; however, the terms are confusing, are not prognostically relevant, and have been abandoned. Pure squamous carcinoma of the endometrium is extremely rare, representing less than 1% of endometrial carcinoma, and with only about 60 reported cases.
Serous
Serous carcinoma of the endometrium closely resembles serous carcinoma of the ovary and fallopian tube because its papillary growth and cellular features are similar. It is usually found in an advanced stage in older women, when comprehensive surgical staging is performed. Serous carcinoma represents about 10% of endometrial carcinomas. The tumors often deeply invade the myometrium, and unlike typical endometrioid adenocarcinoma, there is a propensity for peritoneal spread.
Unfortunately, advanced-stage disease or recurrence is common even when serous carcinomas are apparently only minimally invasive or even confined to the endometrium in polyps. Since the metastatic disease is often identified only microscopically, about 60% of patients are upstaged following complete surgical staging. A recent report by Wheeler et al. stressed the prognostic importance of meticulous surgical–pathologic staging. They and others found that serous carcinoma truly confined to the uterus had an overall excellent prognosis, while those with extrauterine disease, even if only microscopic in size, almost always suffered recurrence and death from tumor.
There has been considerable confusion about the definition and significance of papillary carcinoma of the endometrium. A variety of cell types of endometrial adenocarcinoma with differing biologic behavior, including serous, clear cell, mucinous, and villoglandular carcinoma, may grow in a papillary fashion. Thus, the adjective papillary does not represent a cell type but rather an architectural pattern and should no longer be used in describing serous carcinomas.
Other Cell Types
Clear cell adenocarcinoma of the endometrium represents approximately 4% of all uterine tumors. It is generally recognized and defined on the basis of the distinctive clearing of the cytoplasm of neoplastic cells. These are biologically aggressive neoplasms with 5-year survival of approximately 40% regardless of stage. These results are attributed to a high propensity for extrauterine spread and frequent recurrence. In fact, occult extrauterine metastasis is present in 40% of patients with disease clinically confined to the uterus.
Mucinous adenocarcinoma is rare in the endometrium, representing approximately 1% of endometrial adenocarcinomas. Mucinous carcinoma of the endometrium has the same prognosis as common endometrial carcinoma. If an endometrial carcinoma manifests two or more different cell types, each representing at least 10% or more of the tumor, the term mixed cell type is appropriate.
Malignancies in other organs may metastasize to the endometrium. The most common extragenital sites are the breast, stomach, colon, pancreas, and kidney, although any disseminated tumor could involve the endometrium. The ovaries are the most likely genital sources of metastasis.
Cancers of an identical cell type may be discovered in the ovary and endometrium simultaneously. Usually, the primary site is assigned to the area having the largest tumor mass and most advanced stage. In certain situations, primary malignancies in the endometrium and ovary may coexist. This “field effect” of the “extended müllerian system” may occur in 15% to 20% of endometrioid carcinomas of the ovary. In a review of a GOG study of 74 patients with simultaneously detected endometrial and ovarian carcinoma with disease grossly limited to the pelvis, only 16% of women suffered a recurrence of disease, with a median follow-up of 80 months. This group of patients was atypical, with 86% having endometrioid histology in both sites. Recurrence was statistically related to the presence of microscopic metastases or high histologic grade.
CANCER PREVENTION AND SCREENING FOR ENDOMETRIAL CANCER
KEY POINTS
- All women should be encouraged to promptly report abnormal vaginal bleeding.
- Women taking tamoxifen (usually as breast cancer prevention or treatment) do not require endometrial screening in the absence of symptoms.
Many cases of endometrial cancer develop by way of a precursor lesion. Estrogen-related cancers frequently develop secondary to atypical endometrial hyperplasia. Serous tumors also may develop EIC through a precursor lesion. Prompt recognition of precursor lesions with institution of proper treatment will prevent cancers and their sequelae. Many endometrial cancers may be preventable, particularly those that are estrogen related.
Because 95% of endometrial carcinomas occur in women 40 years and older and because endometrial hyperplasia, the precursor state, tends to be a premenopausal and perimenopausal condition, it is appropriate to evaluate individuals past their fourth decade of life if there is abnormal bleeding. Similarly, a higher degree of suspicion should be held for younger patients with high-risk characteristics including significant obesity, polycystic ovarian syndrome/chronic anovulation, or tamoxifen exposure.
Evaluation can be by endometrial biopsy or by dilatation and curettage (D&C) if the biopsy is unsuccessful or the results are unclear. Patients with complex and atypical hyperplasia may be treated by hysterectomy or by periodic use of progestins, depending on age and reproductive desires. Hysterectomy is the preferred treatment in the patient with complex atypical endometrial hyperplasia. This approach not only cures the usual presenting symptoms of abnormal bleeding but confers prophylaxis against the almost 30% risk of later developing endometrial carcinoma. Since coexistent invasive endometrial cancer may be present in up to 40% of women with CAH, frozen section and surgical staging should be available at the time of hysterectomy.
Those with CAH treated with progestins should have a D&C performed before treatment to rule out the occasional occult carcinoma not detected by biopsy. A progestin should be administered at least 10 to 14 days each month, and endometrial biopsies should be performed at 3- to 4-month intervals to assess treatment results. Women with a uterus should never be prescribed estrogen-only preparations of hormone replacement therapy because unopposed estrogen greatly increases their risk of endometrial cancer. The addition of progestins to the regimens of patients treated with exogenous estrogen may prevent endometrial hyperplasia and subsequent cancer and may protect against the development of carcinoma.
Women taking tamoxifen and have their uterus intact should be informed of the increased risk of endometrial cancer. Education regarding the onset of vaginal bleeding is important, and patients should be advised to report symptoms early. All women on tamoxifen who present with abnormal vaginal bleeding should be investigated promptly. However, screening of asymptomatic women on tamoxifen therapy with ultrasound or endometrial biopsies is not recommended.
Considering the available knowledge about the disease and available tests, it seems unlikely that screening would be generally advised in the near future. This has also been the view in statements from official organizations like the International Union Against Cancer and the American Cancer Society (ACS). The ACS does recommend annual endometrial biopsies starting at age 35 for women known to have or be at risk for HNPCC. Obese patients should be counseled that a healthy diet and regular exercise could reduce their risk of endometrial cancer (in addition to other known benefits).
DIAGNOSTIC EVALUATION
KEY POINTS
- All postmenopausal bleeding should be evaluated.
Endometrioid adenocarcinoma is likely to present with disease confined to the uterus, while serous and clear cell tumors are more likely to have extrauterine spread and preoperative CA-125 and/or imaging is warranted.
Approximately 90% of patients will present with abnormal vaginal discharge, of which 80% will report abnormal bleeding (usually postmenopausal) and 10% show leukorrhea. Other signs and symptoms of more advanced disease include pelvic pressure and other symptoms indicative of uterine enlargement or extrauterine tumor spread (abdominal bloating, pain, shortness of breath).
The standard method of assessing uterine bleeding is formal fractional D&C. Outpatient procedures, such as endometrial biopsy or aspiration curettage coupled with endocervical sampling, may be indicated if the clinical suspicion is sufficiently high. However, sampling techniques may not provide sufficient diagnostic information, in which case the fractional D&C is mandatory. Results of endometrial biopsies correlate well with endometrial curettings.
After the diagnosis of endometrial carcinoma has been histologically confirmed, the patient should undergo a thorough clinical evaluation consisting of a physical examination and careful medical history, complete blood count, liver and renal function tests, and chest x-ray. Further imaging can be guided by the risk of extrauterine spread. In general, women with well-differentiated endometrioid tumors do not need to undergo an extensive metastatic workup. However, patients with high-grade tumors should undergo further evaluation. This may include intravenous including intravenous pyelogram, cystoscopy, and proctoscopy if there is suspicion for extrauterine disease; body imaging by computed tomography (CT) with or without 18F-fluorodeoxyglucose–positron emission tomography (PET); or magnetic resonance imaging (MRI) of the pelvis (particularly indicated if cervical involvement is suspected). In addition, a CA-125 may be useful; if elevated, it becomes a way to evaluate progress during treatment and, subsequently, during surveillance.
Preoperative assessments of lymph node involvement have been proposed by some authors as useful techniques to assess for metastasis. However, only a few pretreatment imaging tests have been described. Both CT and MRI are extensively used to assess nodal spread of disease in patients with malignant tumors, including endometrial cancer. Both techniques have based the detection of pathologic nodes on measurements of node size: a short-axis diameter greater than 10 mm is the most accepted criterion for the diagnosis of suspicious nodal involvement. Unfortunately, these morphologic imaging techniques have low sensitivity ranging from about 20% to 65% with specificity between 73% and 99%.
PET/CT recently has been evaluated for endometrial cancer. Signorelli et al. performed a retrospective study to determine the diagnostic accuracy of 18F-FDG PET/CT in detecting nodal metastases in patients with high-risk endometrial cancer; pelvic node metastases were found at histopathologic analysis in 9 of the 37 patients (24.3%). Patient-based sensitivity, specificity, positive predictive value, negative predictive value, and accuracy were 77.8%, 100.0%, 100.0%, 93.1%, and 94.4%, respectively. Nodal lesion site–based sensitivity, specificity, positive predictive value, negative predictive value, and accuracy were 66.7%, 99.4%, 90.9%, 97.2%, and 96.8%, respectively. From these data, it appears that 18F-FDG PET/CT is accurate in pelvic node staging workup of high-risk endometrial cancer, allowing the best planning of primary surgical treatment in this subset of patients. The GOG is conducting an ongoing prospective assessment of PET/CT in patients with endometrial and cervical cancer (GOG 233).
PATHOLOGIC FACTORS OF PROGNOSTIC SIGNIFICANCE
The importance of uterine and extrauterine risk factors is determined by how they affect the probability of retroperitoneal lymph node involvement and subsequent survival.
FIGO Stage
The prognostic utility of surgicopathologic stage has been confirmed in multiple studies of large numbers of patients, using both univariate and multivariate analyses. FIGO stage is often the single strongest predictor of outcome for women with endometrial adenocarcinoma in studies using multivariate analyses. Although the FIGO clinical staging system of 1971 was generally useful, retrospective comparison of the two methods demonstrated the clear superiority of surgicopathologic staging over clinical staging in predicting outcome (Tables 8.4 and 8.5).
Table 8.5 Grade, Depth of Invasion, and Pelvic Node Metastasis
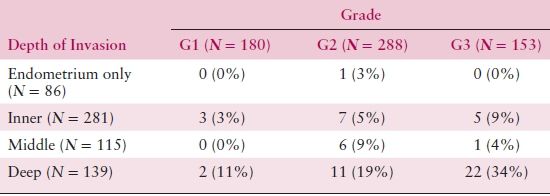
Source: From Creasman WT, Morrow CP, Bundy BN, et al. Surgical pathologic spread patterns of endometrial cancer: A Gynecologic Oncology Group study. Cancer. 1987;60:2039–2041, with permission.
Histologic Cell Types
The histologic classification of endometrial adenocarcinoma is important because it has consistently been recognized as an important predictor of biologic behavior and probability of survival. Endometrioid adenocarcinoma accounts for the majority of tumors in the corpus and, fortunately, usually has a relatively good prognosis.
Adenocarcinoma with squamous differentiation is similar to typical endometrioid adenocarcinoma with respect to the distribution by age and frequency of nodal metastasis but is associated with a slightly increased probability of survival. Villoglandular carcinomas have a biologic behavior similar to that of endometrioid adenocarcinoma. Serous carcinoma is an aggressive tumor, with overall survival (OS) rates varying from 40% to 60% at 5 years. Clear cell carcinoma is also a highly aggressive tumor, with 5-year survival rates of 30% to 75%.
Grade
The degree of histologic differentiation has long been considered one of the most sensitive indicators of tumor spread. The GOG and other studies have confirmed that as grade becomes less differentiated, there is a greater tendency for deep myometrial invasion (Table 8.4) and, subsequently, higher rates of pelvic and paraaortic lymph node involvement (Tables 8.5 and 8.6). In fact, 50% of grade 3 lesions have greater than one half myometrial invasion, with pelvic and paraaortic lymph node involvement approaching 30% and 20%, respectively. Survival has also been consistently related to histologic grade, and in a GOG study of more than 600 women with clinical stage I or occult stage II endometrioid adenocarcinoma, the 5-year relative survival was as follows: grade 1—94%; grade 2—84%; grade 3—72%.
Table 8.6 Grade, Depth of Invasion, and Aortic Node Metastasis
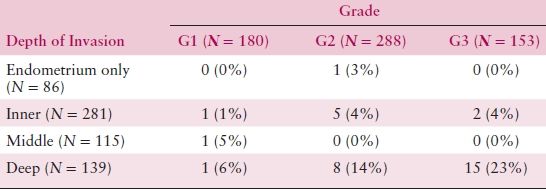
Source: From Creasman WT, Morrow CP, Bundy BN, et al. Surgical pathologic spread patterns of endometrial cancer: A Gynecologic Oncology Group study. Cancer. 1987;60:2039–2041, with permission.
Myometrial Invasion
The depth of myometrial invasion should be recorded in all pathology reports, preferably in both millimeters and in the percentage of total myometrial thickness. Extension of tumor into adenomyosis is not regarded as invasion. Deep myometrial invasion is one of the more important factors correlated with a diminished probability of survival and is associated with a higher probability of extrauterine tumor spread, treatment failure, and recurrence. In a GOG study of over 400 women with clinical stage I and occult stage II endometrioid adenocarcinoma, the 5-year relative survival was 94% when tumor was confined to the endometrium, 91% when tumor involved the inner third of the myometrium, 84% when the tumor extended into the middle third, and 59% when the tumor invaded into the outer third of the myometrium. Although the depth of invasion is often inversely related to the degree of differentiation, myometrial invasion is an independent predictor of outcome for women with early-stage endometrial carcinoma.
Vascular Space Invasion
Several studies have suggested that lymphatic vascular space invasion (LVSI) is a strong predictor of recurrence and death and is independent of depth of myometrial invasion or histologic differentiation. Zaino et al. found that vascular invasion was a statistically significant indicator of death from tumor in early clinical stage but not in early surgical stage endometrial adenocarcinoma. This suggests that lymphatic invasion helps to identify patients likely to have spread to lymph nodes or distant sites, but that its importance is diminished for those in whom thorough sampling of nodes has failed to identify metastasis. Vascular space invasion or capillary-like space involvement with tumor exists in approximately 15% of uteri containing adenocarcinoma. Nodal metastases are more common when capillary invasion is identified. Capillary invasion is identified in 35% to 95% of serous carcinomas of the endometrium, where it has generally been associated with an elevated risk of tumor recurrence or death from disease.
Adnexal Involvement
Six percent of clinical stage I and occult stage II patients have spread of tumor to the adnexa. Of these, 32% have pelvic node metastases, compared with 8% pelvic node positivity if adnexal involvement is not present. Twenty percent have positive paraaortic node metastases, which is four times greater than if adnexal metastases are not present. Gross intraperitoneal spread without adnexal metastases correlates highly with the involvement of pelvic and paraaortic lymph nodes.
Peritoneal Cytology
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


