New levels (as yet unnamed)
Escalating risk of serious adverse event
Physiological monitoring parameters (singular or combination)a
Recommended sedationist skill set
Recommended resourcesb
1
≤1:10,000
Consistent with normal awake pattern and frequency
Ability to observe and interpret the agreed-upon physiological monitoring parameters
Appropriate for risk level
2
1:1,000
← Objective monitoring predicts this level of risk
Skills appropriate for maintaining sedation at this risk level and for rescuing from the subsequent level
Appropriate for risk level
3
1:100
← Objective monitoring predicts this level of risk
Skills appropriate for maintaining sedation at this risk level and for rescuing from the subsequent level
Appropriate for risk level
4
≥1:10
← Objective monitoring predicts this level of risk
Skills appropriate for maintaining a patient at this risk level
Appropriate for risk level
Computer-Assisted Personalized Sedation
An emerging technology called a computer-assisted personalized sedation (CAPS) system has the potential to impact the practice of GI endoscopy, which looks promising [34]. Recently, a CAPS system was introduced in the SEDASYS® System (Ethicon Endo-Surgery, Inc., Cincinnati, OH). (Refer to Chaps. 31 and 38.) With the assistance of anesthesia consultants, SEDASYS was developed, trialed, and subsequently approved by the FDA in 2013. It is expected to be marketed in the United States in the early 2014. It is a CAPS-based intravenous propofol delivery system designed for ASA I and II adult patients undergoing endoscopy. The SEDASYS incorporates computer software that monitors vital signs (pulse oximetry, respiratory rate, capnography) and a patient’s response (appropriate response to verbal command) in order to regulate the delivery of propofol. This was a non-blinded multicenter randomized comparative study (four ambulatory surgery centers, three endoscopy centers, and one academic center in the United States). One thousand ASA I to III class adults were enrolled and underwent routine colonoscopy or endoscopy. Sedation with propofol using the SEDASYS system and sedation with each site’s current standard of care were compared (benzodiazepine/opioid combination). The results showed that the “area under the curve” for oxygen desaturation was significantly lower for the SEDASYS group (23.6 %) versus (88.0 %) for the comparative group p = 0.28. In addition, patients who received propofol were more satisfied, and the recovery was expectedly faster than the comparison group [35]. Currently, the SEDASYS is approved for adults only, and there has not been any clinical application, nor studies performed with it in the pediatric population.
ASA Guidelines, Statements, and Education Modules
In summary, the American Society of Anesthesiology has, over the years, provided guidelines, statements, and education modules (Sedation and Analgesia by Non-anesthesiologists) [36]. This module is available online or as a DVD and includes carbon dioxide basic monitoring and advanced life support information. It also provides basic information and knowledge of sedative and analgesic drugs for moderate sedation. It emphasizes patient safety with proper training in sedation. The educational contents, training, and credentialing of these providers, however, vary from institution to institution. The remainder of this chapter will discuss and review the sedation protocols that have been established and delivered under the auspices of anesthesiologists.
Development of Protocols
Pediatric anesthesiologists have, at their disposal, a wide armamentarium of drugs for sedation. Anesthesiologists administer sedatives and analgesics independently—most commonly without set protocols. Typically, non-anesthesiologists follow protocols, many of which have been developed by anesthesiologists, with respect to sedation administration, monitoring, and recovery [37, 38]. Ironically, albeit anesthesiologists have supported the sedation training and protocol development of non-anesthesiologists, many also oppose the practice whereby non-anesthesiologists can use anesthesia billing codes and deliver propofol [39–42]. This chapter will continue by exploring the variety of protocols that have been established for various commonly administered sedatives.
Ketamine
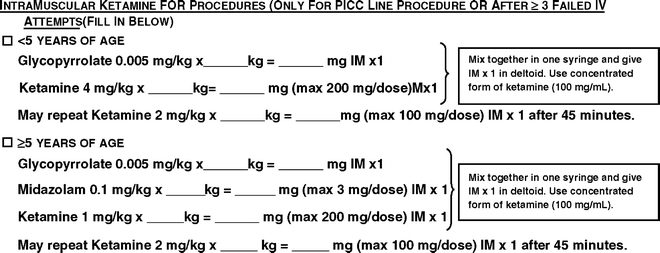
Ketamine has been used as an adjunct analgesic and hypnotic for radiological procedures, hearing tests, endoscopies, fracture reductions, suture insertion and removal, and oncological procedures such as lumbar puncture. Ketamine is versatile because it confers sedation, analgesia, and amnesia. A sedation protocol at Boston Children’s Hospital allows radiology registered nurses (RN) to administer intravenous or intramuscular ketamine (under the auspices of a staff anesthesiologist or radiologist) for interventional, painful radiological procedures such as angiographies, percutaneous gastrostomy tubes, percutaneous inserted central catheters (PIC lines), and organ biopsies. There are clearly defined contraindications to the administration of ketamine, which guide the triage (screening) process (Table 14.2) [29, 43]. The advantage of these protocols was that it provided for the intramuscular (IM) route of administration for children who were not amenable to an intravenous (IV) initiation. This was particularly valuable for children who required a peripherally inserted central catheter (PIC) line for failure to achieve intravenous access. The Boston Children’s Hospital protocol specified IM ketamine (using the concentrated form of ketamine 100 mg/kg) at an initial dose of 4 mg/kg for children under 5 years of age and 1 mg/kg above 5 years of age. Concomitantly, glycopyrrolate at 0.005 mg/kg was added to reduce the increased secretions associated with ketamine. Midazolam was administered to children over 5 years at 0.1 mg/kg to reduce the incidence of nightmares and hallucinations (Fig. 14.1 [29]). An intravenous ketamine protocol was also developed for administration by registered nurses. Ketamine IV 1 mg/kg and IV glycopyrrolate 0.005 mg/kg were administered together for procedures less than 10 min duration. Midazolam IV at 0.1 mg/kg was administered to those who were older than 5 years in order to decrease the incidence of hallucinations. For longer procedures, IV ketamine was delivered as a bolus followed by a continuous infusion of 50–125 mc/kg/min to maintain adequate depth of sedation (see Fig. 14.2 [29]). In oncology patients requiring procedures such as lumbar puncture, IV ketamine at an initial dose of 1 mg/kg (maximum of 75 mg), followed by additional boluses of 0.5 mg/kg as necessary (maximum of 2 mg/kg) was found to be effective [44].
Exclusion criteria for ketamine-induced sedation |
|---|
Contraindications to the use of ketamine |
1. Active pulmonary infection or disease |
2. Known or potential (i.e., risk of) airway compromise |
3. Pulmonary hypertension |
4. Age of 3 months or younger |
5. History of apnea or obstructive sleep apnea |
6. Craniofacial defect that would make mask ventilation difficult |
7. Complex cardiac disease |
8. Acute globe injury |
9. Prior adverse reaction to ketamine |
10. History of bipolar disease or schizophrenia |
11. Head injury associated with loss of consciousness, altered mental status, or emesis |
12. Intracranial hypertension (i.e., CNS mass lesions, hydrocephalus, head injuries associated with increased intracranial pressure); if there is any doubt, please have radiologist consult ordering physician to determine whether there is increased intracranial pressure risk |
13. Any child in whom there is a question of increased intracranial pressure |
14. Child with potential ventriculoperitoneal shunt malfunction |
15. Increased intraocular pressure |
16. Patient or parent refusal |

Fig. 14.1
This protocol is primarily for ketamine use when intravenous access is difficult or not attainable. The age groups are divided into children less than 5 years and children greater than 5 years [29]
Ketamine has also been used as an adjunct with propofol. Some have identified this combination using the term “ketofol” [45]. This combination is intended to decrease the dosing of each sedative, maintain hemodynamics, and decrease the risk of respiratory depression. A comparison of propofol versus propofol-ketamine combination for sedation during spinal anesthesia showed the combination provided better quality sedation with fewer complications as compared to propofol alone [46]. A protocol for auditory testing (ABR) of children age 1–13 years, demonstrated that the combination of 0.5 mg/kg ketamine and 1.5 mg/kg of propofol, decreased the need for additional boluses of propofol when compared to propofol alone (1.5 mg/kg). The ketamine and propofol (ketofol) combination decreased the total amount of propofol used. In addition no patients in the ketofol group had any desaturation or apneic events, whereas four patients had desaturation events and six had apneic events. Additional doses were needed in the propofol group (21) versus (8) in the propofol-ketamine (ketofol) group [47]. A retrospective analysis of an IM ketamine-midazolam-atropine (5 mg/kg, 0.1 mg/kg, 0.01 mg/kg, respectively) combination for ABR sedation showed this combination to be effective with minimal side effects [48]. Another study demonstrated that a combination of propofol and ketamine was very effective for pediatric burn dressing changes [49]. In endoscopic procedures, there seems to be better tolerance during insertion of the scope when a combination of propofol-ketamine is used as opposed to ketamine-fentanyl [49].
Agents such as ketamine and dexmedetomidine have been used in combination for cardiac catheterizations but were not found to be superior to the ketamine-propofol combination with respect to analgesia and sedation conditions. The dexmedetomidine group had increased recovery time and a higher ketamine requirement than did the propofol-ketamine combination [50].
A protocol developed using intranasal sufentanil and ketamine was developed in Europe. The bioavailability of sufentanil and ketamine was 24.6 % and 35.8 %, respectively. A low dose of 0.5 mcg/kg intranasal sufentanil and 0.5 mg/kg ketamine was effective in 78 % of children undergoing painful procedures [51].
Pentobarbital
Although the drug has been in clinical use for more than 150 years and has an established safety record for sedation, the limitation is its relatively long half-life ranging between 15 and 48 h [52]. Pentobarbital given by the oral route (up to 8 mg/kg) has been shown to have a lower incidence of adverse events as compared to oral chloral hydrate [30]. An advantage of pentobarbital is that it may be administered by the oral and intravenous route with similar efficacy and adverse event profiles for each route [30]. Boston Children’s Hospital still continues to have a nursing-administered pentobarbital sedation service. With clearly defined protocols, pentobarbital by the intravenous or oral route has been shown to be efficacious, predictable, and relatively safe. Although it has no analgesic properties, in conjunction with judicious administration of a narcotic, it may be used successfully for painful procedures [30, 53–55].
Dexmedetomidine
Dexmedetomidine is a selective alpha-2 adrenergic agonist that was approved in the United States in 1999 for intubated adults, and then in 2010 for the sedation of adults in areas outside of the operating room and ICU. It is not approved for pediatric usage although it is being used widely for this population in clinical practice. It was recently approved in September 2001, in Europe, for sedation in the ICUs. Although it does not carry pediatric labeling anywhere in the world, it has demonstrated itself to be effective and respiratory sparing as a sedative for non-painful radiological imaging studies, as well as a useful perioperative adjunct to improve analgesia and decrease emergence delirium [12, 54, 56–61].
Between 2005 and 2010, dexmedetomidine was incorporated into the nursing-administered sedation program for MRI at Boston Children’s Hospital. It still continues to be administered by nurses for computerized tomography (CT) sedation, under the auspices of a physician from the Department of Anesthesia [54, 57–59, 61]. Clearly defined protocols have been established to guide administration: a list of contraindications to dexmedetomidine guided the triage/screening process (Table 14.3 and Fig. 14.3) [59]. An initial loading dose of 2–3 mcg/kg IV dexmedetomidine is administered over a 10-min period. Using the Ramsay Sedation Scoring System, the child would receive an additional bolus of 1–2 mcg/kg IV over another 10 min if he/she failed to achieve a Ramsay Sedation Score (RSS) of 4 after the first bolus. Once the child achieves this intended level of sedation, the sedation is maintained with an infusion dose of 1–2 mcg/kg/h. The infusion is stopped once the procedure is completed. The patient is then transported to a recovery area until discharge criteria (based on a modified Aldrete score) are met [58, 60]. In approximately 10 % of the cases, there was a need for adjuvant sedation with up to 2 mg/kg IV pentobarbital for optimal imaging conditions [60]. At the University of Iowa and St. Louis Children’s Hospital, the dexmedetomidine protocols from Boston Children’s Hospital have been adapted to include IV midazolam 0.1 mg/kg, IV ketamine 0.5 mg/kg, and propofol as alternatives to pentobarbital.
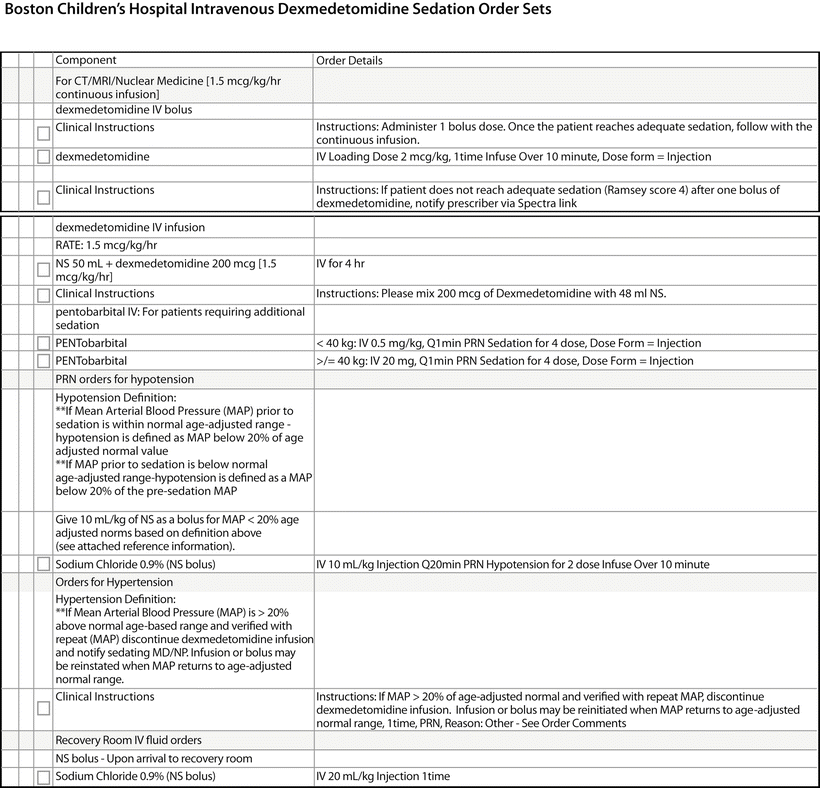
Table 14.3
Medical conditions that contraindicate dexmedetomidine and nursing-administered sedation [59]
Medical conditions that contraindicate dexmedetomidine and nursing-administered sedation |
|---|
1. Active, uncontrolled gastroesophageal reflux—an aspiration risk |
2. Active, uncontrolled vomiting—an aspiration risk |
3. Current (or within past 3 months) history of apnea requiring an apnea monitor |
4. Active, current respiratory issues that are different from the baseline status (pneumonia, exacerbation of asthma, bronchiolitis, respiratory syncytial virus) |
5. Unstable cardiac status (life-threatening arrhythmias, abnormal cardiac anatomy, significant cardiac dysfunction) |
6. Craniofacial anomaly, which could make it difficult to effectively establish a mask airway for positive pressure ventilation if needed |
7. Current use of digoxin |
8. Moyamoya disease |
9. New-onset stroke |

Fig. 14.3
Boston Children’s Hospital intravenous dexmedetomidine sedation order sets
Propofol
Propofol is not FDA-approved as a sedative, but rather is considered an anesthetic agent [62]. In general, its administration should be reserved to only those who are skilled in the administration, recognition, and rescue from general anesthesia. Both the American Association of Nurse Anesthetists and American Society of Anesthesia made a joint statement on the need for restricting the use of propofol [63]:
Whenever propofol is used for sedation/anesthesia, it should be administered only by persons trained in the administration of general anesthesia, who are not simultaneously involved in these surgical or diagnostic procedures. This restriction is concordant with specific language in the propofol package insert, and failure to follow these recommendations could put patients at increased risk of significant injury or death.
Though the controversy on the use of propofol by non-anesthesia providers remains—especially in the United States—there is an increasing group of providers using it for sedation for endoscopic procedures [64].
Propofol protocols for upper gastrointestinal (GI) endoscopies have been developed in conjunction with other medications such as tramadol (used instead of fentanyl), which confers better respiratory stability than does fentanyl. A baseline level of sedation was provided with 1 mg/kg, and then patients were randomly assigned to receive fentanyl at 2 mcg/kg or tramadol at 2 mg/kg. It was found that tramadol provided sedation as efficient as fentanyl with better hemodynamic and respiratory stability (using propofol in the background as a steady dose) [65]. A meta-analysis of nine randomized controlled trials compared propofol in combination with other sedatives (such as midazolam, remifentanil, alfentanil, meperidine, fentanyl, and etomidate) to propofol alone. Propofol in combination was shown to decrease the total dose of propofol without affecting any change in cardiopulmonary complications [66]. Though the controversy of propofol use continues, the popularity of its use continues to grow with non-anesthesia providers. The recent first CAPS device using propofol was approved by the FDA in 2013 for commercial distribution in 2014 [67]. It has only been approved for use in healthy adult ASA I and II patients age 18 years and older. Although intended to allow non-anesthesiologists, gastroenterologists in particular, to deliver propofol for adult endoscopies, the FDA specifies that it requires the immediate availability of anesthesia [68]. The definition of “immediate availability” is not defined and is left to the discretion of the providers, institution, or facility.
A Close Examination of Some Unique Sedation Programs Developed in Conjunction with Anesthesia in the United States
Nursing-Delivered Propofol: The University of Iowa
Nursing-delivered propofol has been controversial because its use needs to be restricted (according to governing bodies such as the American Society of Anesthesiology and the FDA) to professionals trained in performing general anesthesia [69, 70].
The University of Iowa has a unique propofol model: registered nurses deliver propofol under the supervision of an anesthesiologist. A sedation protocol for endoscopy of children <10 years of age specifies a 10:1 combination of propofol and ketamine. The ketamine was intended to provide some analgesic and propofol sparing effect. Ketamine is not used as an adjunct to propofol for procedures in children >10 years of age for fear of hallucinations, nausea, and vomiting. Initially, the dosing interval between boluses was at 1 min intervals and slowly reduced to 15 s after the nurses gained experience. There are order sets that detail the dosing (see Table 14.4).
Table 14.4




Propofol protocol for RN sedation (Merete Ibsen, MD, Department of Anesthesia, Carver College of Medicine, University of Iowa)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



