Fig. 9.1
Pre-operative x-ray (a) and MRI (b) demonstrating an aggressive sarcoma of the proximal humerus in a 5-year-old boy. A vascularized fibula (c) was harvested and used to reconstruct the proximal humerus (d)
One additional scenario that deserves discussion is the presence of pathological fracture. The occurrence of a pathologic fracture, either at presentation or during induction chemotherapy, was traditionally treated with immediate amputation. However, it has now been established that although overall survival is worse in these patients (55 % compared to almost 80 %), limb salvage can still be performed in select cases in which negative surgical margins can be achieved (Scully et al. 2002).
9.1.2 Role of Skeletal Maturity
One of the major differences when considering limb salvage versus amputation in the pediatric population is the growth potential in terms of skeletal maturity. Skeletal maturity (as defined by closure of the growth plates) is gender dependent but is generally not reached until the age of 16–18 years. The most common locations for the development of osteosarcoma include the areas of rapid bone growth (distal femur, proximal tibia, proximal humerus). Therefore, the younger the child is at the time of diagnosis, the more growth that must be accounted for in the surgical decision and reconstruction. In general, the younger (and smaller) the patient, the more difficult limb salvage is to achieve and the worse the morbidity (Guillon et al. 2011). Additional factors that play a role in the decision to perform limb salvage in the skeletally immature patient include size of the resection and tumor, size of available implants, other reconstructive options, size of soft tissue envelope, and potential for lengthening after index procedure.
9.2 Indications and Contraindications for Limb Salvage
With current treatment approaches, limb salvage can generally be offered in 90 % of cases (Wilkins et al. 2005). General indications include isolated disease, a good response to chemotherapy, and no involvement of the adjacent neurovascular structure. There are really no absolute contraindications to limb salvage, but the overall function of the limb should always be considered and several relative contraindications have been defined. These contraindications to limb salvage include progression during chemotherapy, encasement of neurovascular structures, the very skeletally immature patient, and sometimes pathologic fracture resulting in a high degree of local tissue contamination. Another consideration should be minimizing the time to resumption of chemotherapy after the local control surgery. Outcome data suggest that systemic therapy should be resumed within 6 weeks of surgery to maximize survival for the patient. Therefore, attempts to minimize infection and wound complications should be paramount. Judicious use of flaps for wound coverage, minimizing operative time, and pre-surgical optimization of blood counts should all be employed to minimize the time the patient will be off systemic treatment.
9.3 Reconstructive Options
Once the decision to proceed with limb salvage has been made, there are multiple potential options available for the reconstruction. When the tumor is present in an expendable bone, local control can be achieved with resection without reconstruction. Locations amenable to this include the fibula, portions of the pelvis and sacrum, and portions of the ulna. Outside of these select areas reconstruction is usually required.
9.3.1 Autograft
The use of autografts in reconstruction for structural defects is fairly limited. The most common indication utilizes the fibula, either in a vascularized or non-vascularized fashion. The mid-portion of the fibula can serve as a structural strut, and has been described in combination with an allograft to promote healing (Li et al. 2011). Vascularized fibular graft has also been described for reconstruction of intercalary resections of long bones (tibia, femur, humerus) (Chen et al. 2007). Another potential indication exists in the very young child (<8 years old) with a malignant proximal humerus tumor. Skeletal growth is an issue, and the proximal fibular epiphysis can be harvested on a vascularized pedicle for reconstruction in an attempt to maintain some growth in the limb (Fig. 9.2).
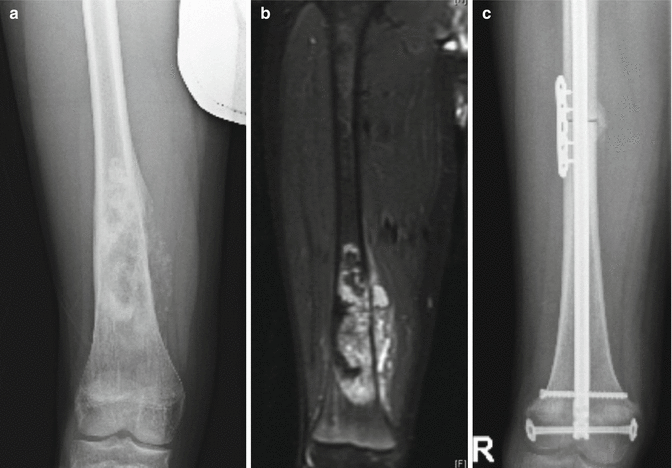

Fig. 9.2
Pre-operative x-ray (a) and MRI (b) showing an osteosarcoma of the distal femur. Intercalary physeal sparing allograft was used to reconstruct the defect (c)
9.3.2 Allografts
Prior to the advent and development of metallic prosthesis in the last 30 years, allograft reconstruction was the mainstay of treatment for pediatric sarcoma patients. Allografts have been used longer than any other reconstruction method. Osteoarticular allografts were used extensively for reconstruction involving the joint and allow a strong repair for ligaments and other soft tissue. Unfortunately, very few of the chondrocytes survive the preservation and sterilization process, so the natural course is for the joint surface to degenerate over time. Osteoarthritis develops in the first 5–10 years in 15 % of patients (Mankin et al. 1996). Thus, metallic solutions have largely replaced this reconstruction when tumor resection requires the joint to be removed, although it can still be employed in select circumstances (Muscolo et al. 2008).
The major usage of structural allografts occurs in intercalary resections and reconstructions (Fig. 9.3). This technique can be employed in cases where the tumor is limited to the diaphysis and the physis can be spared. The allograft is usually stabilized and protected with either a plate or rod, which minimizes the potential for fracture of the allograft (Miller and Virkus 2010). The ability to spare the joint allows functional outcomes to be maximized for these patients.
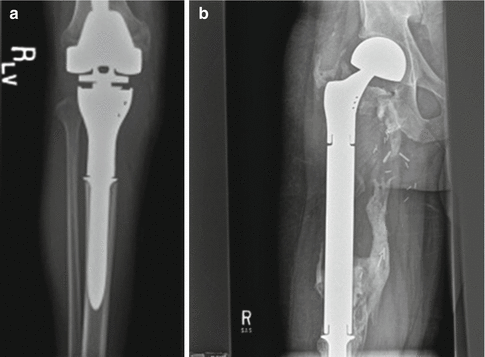

Fig. 9.3
Examples of modular prosthesis. A proximal tibia (a) and proximal femur (b) is shown. Heterotopic ossification is commonly seen after the reconstruction, seen particularly well in (b)
The modes of failure associated with allograft reconstruction include infection, fracture, and non-union. Up to 50 % of patients can be expected to experience one of these complications, and the rate of early complication is high (Mankin et al. 1996).
9.3.3 Metallic Prosthesis
The advent and development of metallic “mega”-protheses has replaced the utilization of allografts in most circumstances. Modern systems offer tremendous modularity that allows the surgeon to reconstruct almost any length of skeletal defect intra-operatively (Fig. 9.4). Immediate range of motion and weight bearing can often be allowed with these constructs. In addition, there is a lower risk of infection and non-union when compared to allografts, and there is no risk of disease transmission. These devices can also be used for salvage after failure of a prior allograft (Foo et al. 2011). Also available are expandable prostheses, which allow growth of the limb in the child in either an invasive or non-invasive manner (Fig. 9.5). The development of expandable options was a tremendous advance in the realm of limb salvage for young patients (Beebe et al. 2010). The child usually undergoes lengthening every 1 cm or so. Lengthening in larger increments can cause increased pain and neuropraxia. Despite high interest and emotional acceptance with these devices, studies have not shown superiority to limb salvage versus amputation (Henderson et al. 2012).
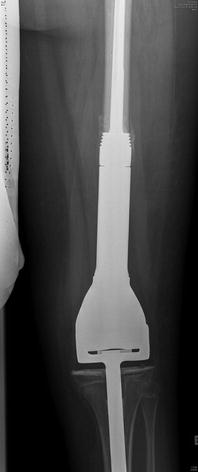
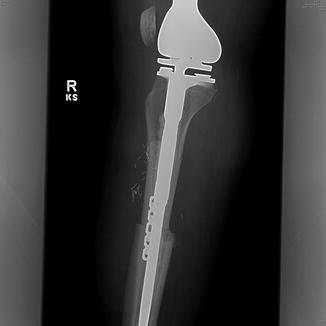

Fig. 9.4
Plain radiograph showing reconstruction with a non-invasive expandable prosthesis in a 8 years old patient. The prosthesis can be lengthened in the office using an electromagnetic coil

Fig. 9.5
Lateral radiograph of a knee showing an example of an allograft-prosthestic combination reconstruction that was utilized after resection of the proximal tibia for osteosarcoma. An allograft proximal tibia with extensor mechanism attached is used to reconstruct the bone defect and a metallic prosthetic knee is placed in combination for joint reconstruction
The main modes of failure for metallic prosthesis include loosening and infection. Either of these can be disastrous for the patient and can lead to extensive revision or even to amputation. The 10-year survival for these devices is around 60 % (Morgan et al. 2006). Location plays a role in the durability of the reconstruction, with proximal humeral and proximal femur doing the best (77 % at 10 years), distal femur next (65 % at 10 years), and proximal tibia prostheses faring the worst (50 % at 10 years) (Damron 1997).
9.3.4 Combination (Alloprosthetic Composite)
Combining both an allograft and a prosthetic in a reconstruction is performed in an attempt to capture the benefits and avoid the pitfalls of each of the respective methods. The composite reconstruction can help restore bone stock and provide strong ligamentous attachments with the allograft portion, while the prosthetic portion avoids the cartilage degradation associated with osteoarticular allografts (Fig. 9.6).
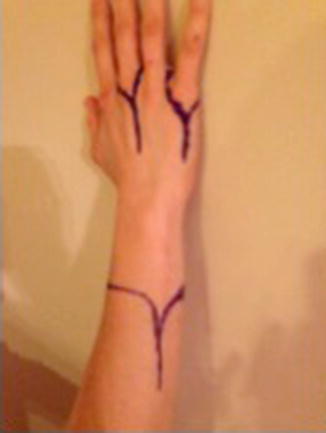

Fig. 9.6
Incisions for transradial amputation and ray resections
9.4 Amputation
Amputation is one of the oldest surgical procedures known to man. The most common indications for amputation are: infection, ischemia, trauma, and malignancy. With improved antibiotics, revascularization procedures, soft tissue reconstruction, and limb-salvage techniques, fewer patients require amputation today than ever before. Nevertheless, sometimes amputation is the more prudent option in terms of either time for recovery or risk of complications. In these instances, it is important for surgeons to emphasize to patients that amputation represents the beginning of a new life rather than the culmination of previous treatment failures (Figs.9.7 and 9.8).
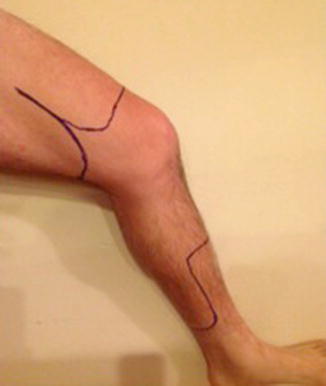
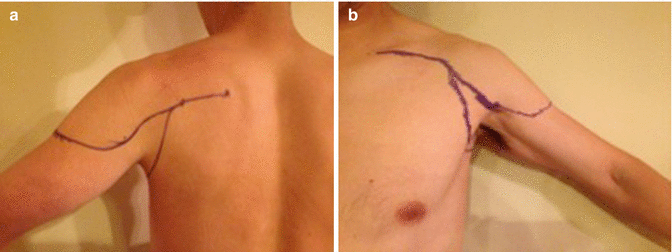

Fig. 9.7
Incisions for above- and below-knee amputations

Fig. 9.8
Incision for shoulder disarticulation or forequarter amputation. (a) Posterior incision (b) Anterior incision
9.4.1 Indications
Approximately 13,000 Americans live with lower extremity amputations due to malignancy (Ziegler-Graham et al. 2008). With the advent of modern chemotherapy, survival rates have improved from approximately 20 % prior to the 1970s to nearly 70 % for primary bone sarcomas (Ng et al. 2013). Effective imaging technology and radiation techniques have allowed most patients with soft tissue sarcomas to preserve their limb without significant increases in local recurrence rates or overall mortality.
The threshold to perform an amputation for tumor varies widely amongst institutions. Every situation is unique and should be evaluated on a case-by-case basis. There are numerous important factors to consider (Table 9.1).
Table 9.1
Considerations impacting surgical choice for malignant bone tumors
Patient factors | Oncologic factors |
Risk tolerance for recurrence | Responsiveness to adjuvant treatment |
Occupation | Local aggressiveness |
Ability to undergo rehabilitation | Stage of disease |
Age | Single lesion or multifocal lesion |
Cosmesis
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|