Specimen Collection and Processing
Portio, Vagina, and Endocervix
| 6 | Specimen Collection and Processing |
Portio, Vagina, and Endocervix
Cytology
Taking a smear for early detection of cervical cancer is probably the most important routine procedure carried out in a gynecological practice, because it can effectively prevent the development of cervical carcinoma. In Germany, this is considered a screening examination, meaning that it is carried out on clinically healthy women at least once a year and is paid for by the health insurance system.
The utmost care should be taken with such an examination, as any avoidable error during cell sampling may have legal consequences for the physician. Although the correct and efficient sampling of smear material should be an essential part of medical specialist training, it frequently happens that the quality of smears is unsatisfactory and the cytology laboratory has to advise office-based physicians on the correct sampling techniques.
The cells are sampled from the portio vaginalis or, in women with a history of hysterectomy, from the fornix of the blind vaginal pouch.
Prerequisites for Cell Sampling
Unfavorable conditions. The menstrual phase is not a suitable time for taking a smear, because blood, cell debris, leukocytes, and endometrial cells make it difficult to establish a diagnosis. Inflammation also has an unfavorable effect on the assessment and, if possible, should be treated before a smear is taken.
Although postmenopausal atrophy of the vagina is not an optimal condition, it is nevertheless often unavoidable because general hormonal therapy before cell sampling would be too uneconomic.
No medication should be applied intravaginally within 1–2 days before the smear sample is taken. Any interventions in the region of the portio vaginalis—such as cauterization, thermocoagulation, cryotherapy or laser therapy, polypectomy, curettage or conization—should occur more than 1 month before sampling. This interval also applies to sampling after delivery.
Favorable timing. Sufficient supply of estrogen to the squamous epithelium provides optimal preconditions for cell sampling, since it ensures differentiation of the cervical epithelium up to the superficial cell layer. During sexual maturity conditions are optimal in the middle of the cycle; after the menopause, they need to be induced by hormonal replacement therapy or topical estrogen application.
Site of cell sampling. The site of cell sampling, in addition to proper timing, is decisive for the efficiency of smear collection. Since about one third of all cervical carcinomas develop within the cervical canal, it is important to sample not only cells from the squamous epithelium of the portio vaginalis but also cells from the columnar epithelium of the endocervix.
Sampling Devices
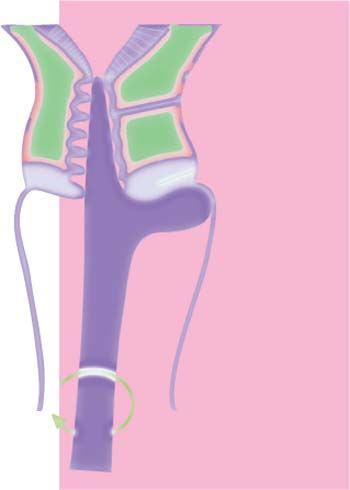
Numerous different instruments are available for sampling cervical cells (Fig. 6.1). They are usually more effective when used assertively rather than in a way that is less damaging to the tissue.
Cotton swab. For a long time, until other devices were developed, cell sampling with a cotton swab was considered inexpensive and suitable. Newer techniques ensure that the specimen obtained is not only better preserved but also contains endocervical cells. In this respect a spatula is superior to a cotton swab (322). Because of its softness, the cotton swab only picks up cells already exfoliated from the surface of the tissue and subjected to cytolytic digestion by the vaginal secretion. The spatula, being much harder, removes cells from the superficial tissue layer, which are therefore preserved in good condition. Another disadvantage of the cotton swab is that it often does not penetrate sufficiently into the endocervix. As a result, no columnar epithelial cells—or not enough of them—are harvested if the transformation zone between squamous epithelium and columnar epithelium lies inside the cervix.

- plastic spatula (Szalay Cyto Spatula)
- endocervical brush (Cytobrush)
- cervical broom (Cervex Brush)
- platinum loop
- wooden spatula (Ayre spatula)
- cotton swab
Spatula. The spatula has developed from a simple wooden spatula (Ayre spatula) to the more efficient plastic spatula (e.g., the Szalay Cyto Spatula). The latter has a rough surface to ensure strong cell adhesion. Furthermore, the Szalay spatula has a tapered tip that permits safe penetration into the endocervix. There may be a slightly greater tendency for the epithelium to bleed when a spatula is used rather than a cotton swab; however, this is acceptable in view of the considerable advantages of the spatula method. The sampling does not cause pain, provided it is done by an experienced practitioner. There are no clear data available as to whether a plastic spatula may be safely reused; this depends essentially on its suitability for disinfection and sterilization.
Cervex Brush and Cytobrush. Other devices that also aim to collect sufficient amounts of endocervical cells include the cervical broom (Cervex Brush) and the endocervical brush (Cytobrush).
- The Cervex Brush consists of plastic lamellae of various lengths, with the shorter ones designed for gliding over the ectocervix and the longer ones for penetrating into the cervical canal. The disadvantage of this broom-shaped instrument is that large amounts of mucus may get stuck between the plastic lamellae, thus interfering with the mounting and evaluation of the smear.
- The Cytobrush consists of a conical sampler shaped like a bottle-brush. It is introduced into the cervix with a swirling movement, like a pipe cleaner. This device has the disadvantage of sampling only endocervical cells but no squamous epithelial cells. Furthermore, the fine nylon bristles often cause mechanical damage to the sensitive columnar epithelium, thus making evaluation of the smear difficult.
Other sampling devices, such as glass pipettes and platinum loops, no longer play a role these days. Some techniques, e. g., blind sampling with a tampon or the examination glove, have become obsolete because of their lack of accuracy.
Cell Sampling Method
Inserting the speculum. The routine gynecological examination consists of two parts: the visual assessment and the palpatory exploration of the pelvis. Pelvic examination usually introduces glove powder and lubricant into the vaginal cavity and wipes off any cells accumulating on the portio vaginalis. The visual part of the examination should therefore be done first. For this purpose, a specially formed metal speculum is inserted into the vagina to fully expose the portio vaginalis. Any interfering accumulation of mucus or discharge is carefully removed from the portio by means of a cotton swab.
Collecting the smear material. A suitable instrument, such as the Szalay Cyto Spatula, is introduced into the cervical canal until the shoulder of the spatula comes to rest on the surface of the portio vaginalis. The spatula is rotated once or twice in one direction to collect cells (Fig. 6.2a). It is then retracted, and any adherent mucus is carefully removed with absorbent paper. The collected cells are then transferred onto a microscope slide already prepared with the patient’s name on its frosted part. The cells are evenly spread across the slide by guiding the spatula tangentially. While doing so, it is important that the shoulder and tip of the spatula maintain the direction of movement, thus keeping ectocervical and endocervical cells separated (Fig. 6.2b). To increase the yield of endocervical cells, the tip of the spatula may then be rolled over the corresponding area on the slide.
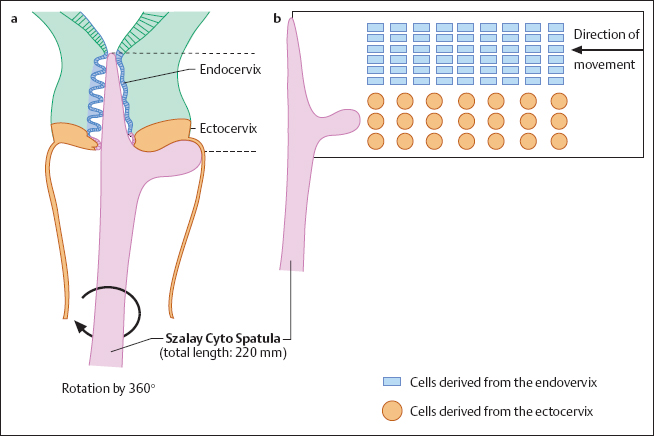
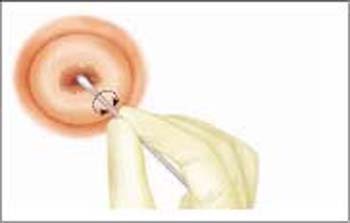
Fig. 6.3 Using a cotton swab for sampling cervical cells. The sampling from ectocervical and endocervical areas should be carried out with two separate cotton swabs. Although this type of cell sampling is no longer commonly used, the technique may be indicated in exceptional cases (e. g., severe atrophy of the genitals associated with reduced exfoliation of cells). The cotton swab should be rolled across the microscope slide to increase the yield of cells.
Difficult sampling conditions. In rare cases (e. g., severe atrophy, or scarring due to conization) the tip of the spatula does not completely penetrate into the cervix. If this is the case, the spatula should only be introduced as far as possible without using force or causing pain. The material collected in this way is transferred onto one half of the slide. The surface of the portio is then wiped with a cotton swab or regular spatula (Fig. 6.3), and the collected material is rolled or smeared onto the other half of the slide.
If a cervical ectopy is present and it is larger than the shoulder of the spatula, the sampling yields only cells derived from the endocervical epithelium. As a result, the smear cannot be evaluated because cells from the squamous epithelium are absent. If this is the case, additional cells from the squamous epithelium should be collected from the peripheral borders of the ectopy using a cotton swab or regular spatula. However, in the case of an ectopy, it is impossible for the cytologist to determine the origin of columnar cells because the glandular epithelium is localized on both ectocervix and endocervix.

In the present example, the findings are reported as Pap classes according to the revised Munich nomenclature (Munich II) used in most European laboratories.
Fixation
The Papanicolaou stain commonly used in cytodiagnostics requires immediate fixation of the smears (within a few seconds after sampling), since even short-term exposure to air causes artifacts that interfere with establishing a diagnosis.
Spray fixation. The fastest and simplest method is to use a spray fixative.
Fixation by immersion.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree