13.1 Host and environmental factors
The neonate may be exposed to skin trauma during delivery, through mechanical pressure (e.g. cephalhaematoma) or instrumentation (e.g. forceps, ventouse, scalp electrodes). The skin of extremely pre-term neonates is fragile and easily damaged and a potential source of infection. The use of skin barrier therapy to prevent infections is discussed in Section 23.3.2. The umbilical cord represents a potential source of infection, preventable with good umbilical cord care (see Section 23.3.1). For infants needing neonatal intensive care, the skin barrier is broken for vascular access and sometimes for invasive procedures such as chest drains.
Surgical procedures including male circumcision can become infected. In the past, the moel who performed ritual Jewish circumcisions achieved haemostasis by sucking the end of the penis, which was not infrequently associated with transmission of Mycobacterium tuberculosis.1 Tuberculosis of the penis associated with circumcision still occurs rarely.2 Other infections associated with circumcision include bacterial infections due to Staphylococcus aureus, GBS, fungal infections3 and acute infections with HSV and Treponema pallidum.4–6 Interestingly, although HSV and syphilis were sometimes acquired at the time of circumcision historically, circumcision actually protects against the acquisition of HSV-2 infection and syphilis later in life.7 Circumcision also protects against urinary tract infections (see Chapter 10),8 HIV infection9 and probably against human papillomavirus (HPV) infections.10,11
Male newborn infants are more susceptible than female infants to colonization with S. aureus and to develop infections.12,13 A review of studies found that males were about 50% more likely than females to develop staphylococcal skin lesions.13
13.2 Organism factors
Some organisms have a predilection for causing skin and soft infections, of which the commonest by far is S. aureus.14–16 MRSA is associated with skin and soft tissue infections in children and adults, notably the USA 300 clone in North America which has been associated with community-acquired MRSA infections.17 Some reports suggest MRSA does not cause more skin and soft tissue infections than methicillin-sensitive S. aureus in neonates.14–16 However, these studies were either under-powered or did not distinguish hospital-acquired from community-acquired MRSA.14–16 Hospital-acquired MRSA typically caused isolated cases or epidemics of septicaemia in neonatal units associated with respiratory and gastrointestinal colonization without significant skin and soft tissue infections.18 A report from Texas showed increasing numbers of community-acquired S. aureus infections in previously healthy term and near-term neonates, with 61 of 89 infections (68.5%) caused by MRSA and 86.5% involving skin and soft tissue.19 Almost all the MRSA belonged to the USA 300 clone and were positive for the Panton–Valentine leukocidin (PVL) gene, which has been associated with skin and soft tissue infections and more severe invasive diseases.19 If PVL-producing MRSA (and MSSA strains which can also carry the PVL gene) become more prevalent in the community, the incidence of skin and soft tissue infections will probably increase.20
Other organisms known to be associated with skin and soft tissue infections include GBS, which can cause neonatal cellulitis, and organisms associated with cellulitis in older children and adults but only rarely in neonates such as GAS (Figure 13.1a).
13.3 Epidemiology
Definitions of skin infections lack precision and epidemiologic data on skin infections are not always reported with precision. Furthermore, some infants with skin infections do not present until after discharge from hospital. The rarity of GAS and Haemophilus influenzae type b (Hib) skin infections in neonates is presumably due to uncommon exposure, although it is also possible that the host immune response contributes to the cellulitis and that neonates are protected against cellulitis by the relative immaturity of their immune response.
13.4 Clinical manifestations
13.4.1 Cellulitis
Cellulitis is a diffuse, spreading skin infection, and the term should exclude infections associated with underlying suppurative foci, such as cutaneous abscesses, necrotizing fasciitis, septic arthritis and osteomyelitis.21 Spreading, tender erythema may be associated with one or more of fever, lymphangitis, lymphadenopathy and systemic toxicity. As the rash progresses, blistering may occur.21 Cellulitis is rarely reported in the neonatal period and the presence of cellulitis should always alert the clinician to the possibility of underlying osteomyelitis or septic arthritis (see Figure 9.5b). Orbital cellulitis can be the presentation of underlying ethmoid osteomyelitis,22 while maxillary osteomyelitis can mimic orbital cellulitis.23
Cellulitis due to GBS is characteristically facial submandibular cellulitis with associated adenitis.24–26 This is a late-onset manifestation of GBS commonly associated with GBS bacteraemia and presenting at a mean age of onset of five weeks with marked local swelling and erythema (Figure 13.1b), poor feeding and irritability. In one report, four of five infants with facial or submandibular GBS cellulitis had ipsilateral otitis media at the time of admission and the authors postulated that primary otitis media with subsequent lymphatic spread to facial or submandibular areas could be the pathogenesis. GBS can cause cellulitis elsewhere, including the inguinal region.27
GAS is an occasional cause of neonatal cellulitis (Figure 13.1a),28 including facial cellulitis: in one series of streptococcal facial cellulitis, five of six cases were due to GBS and the other due to GAS.25 Hib can cause a vesicular eruption at birth although not the classical facial cellulitis of older children, which is often buccal or peri-orbital.29 Gram-negative bacilli are rare causes of cellulitis: one of 14 infants with Serratia infections secondary to contaminated shampoo had cellulitis.30 Other organisms reported to cause occasional cases of cellulitis are Candida which can cause burn-like truncal erythema (Figure 13.1c)31,32 as well as more classic rashes in diaper and intertriginous areas, and anaerobes which can be found in scalp cellulitis secondary to scalp electrodes.33
Cellulitis of the scalp secondary to scalp electrodes may be due to HSV, (see Chapter 17) even if there is bacterial secondary infection.34,35 HSV cellulitis is characterized by discrete punched out lesions (Figure 13.1d) which may coalesce and become secondarily infected making the diagnosis difficult (Figure 13.1e). The diagnosis is a vital one because of the danger of rapid dissemination of HSV.
Cellulitis of the abdomen should alert the physician to a possible diagnosis of necrotizing fasciitis (Section 13.4.2). Empiric treatment of cellulitis should include both anti-staphylococcal and Gram-negative cover given parenterally until blood culture results are back because of the relatively high rate of associated bacteraemia. US clinicians often suggest aspirating the leading edge of an area of cellulitis after infusing saline subcutaneously.
13.4.2 Necrotizing fasciitis and myonecrosis
Necrotizing skin and soft-tissue infections are deeper than cellulitis and may involve the fascial compartment (necrotizing fasciitis) or the muscle compartment (myonecrosis).21 They cause major tissue destruction and have a high mortality. Extensive necrosis is called gangrene.21
A review of the world literature in 1999 yielded 66 cases of neonatal necrotizing fasciitis.36 Only 3 infants were pre-term. Underlying conditions that might have contributed to the development of necrotizing fasciitis included omphalitis (47 or 71%), breast abscess (5), balanitis (4), foetal scalp monitoring (2), necrotizing enterocolitis (1), immune deficiency (1) and bullous impetigo (1). The most common site was the abdominal wall (53 or 80%), followed by the thorax (7), back (2), scalp (2) and extremity (2). The initial skin presentation ranged from a minimal rash to erythema, oedema, induration or cellulitis and spread was characteristically rapid. The overlying skin appearance was variously described as violaceous, peau d’orange, bullae or necrosis. Crepitus was rare. Pain, which is typical of the disease in older children and adults, was hard to evaluate. Fever and tachycardia were common but not invariable. Most infants had neutrophil leukocytosis and half had thrombocytopenia. Of the 53 wound cultures available for bacteriologic evaluation, 13 had a pure growth of one organism, 39 were polymicrobial and one was sterile. Among the 39 specimens with polymicrobial infections, the predominant aerobic bacteria were S. aureus, Escherichia coli and enterococci, and the predominant anaerobes were Clostridium and Bacteroides. S. aureus was the most common organism recovered from the wound cultures with one organism. Blood culture was performed in 40 cases and was positive in half, 15 with a single organism, and 5 polymicrobial. There were 13 episodes of bacteraemia caused by organisms identical to those found in the wound cultures. Two blood cultures and one wound culture grew Candida. The mortality rate was 59% (39 of 66). Twelve of the 27 survivors needed skin grafting because of poor healing or large post-operative skin defects.36 A later case was associated with a PVL-producing strain of methicillin-sensitive S. aureus.37
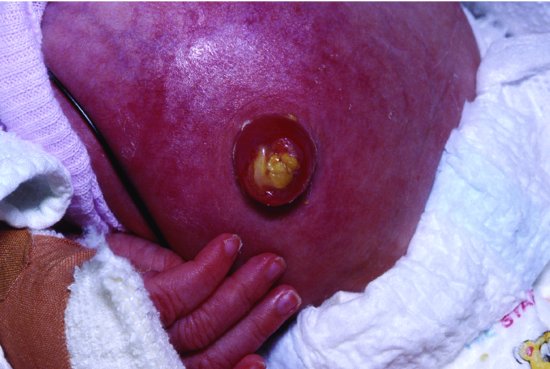
13.4.3 Omphalitis
S. aureus has caused nursery outbreaks of skin infections (bullous impetigo) and umbilical infection (omphalitis) in the United States38 and United Kingdom39 since at least the 1920s. The introduction of skin and umbilical cord care using antiseptics and topical antibiotics to the cord in the 1960s and 1970s was associated with a marked reduction in staphylococcal skin infections and omphalitis. A sequential US study40 showed that staphylococcal colonization and infection were low when babies had total body bathing with 3% hexachlorophene but increased dramatically (80% colonization, 9.5% infection) when hexachlorophene was discontinued and replaced by Ivory Soap baths. Reinstitution of hexachlorophene reduced colonization and infection, although not to the initial low levels. A second Ivory Soap period (period 4) was associated with a return to high rates of colonization (77%) and infection (11.5%). Subsequently, when daily Ivory Soap baths were continued but topical bacitracin ointment was applied regularly to the umbilicus, colonization fell to 10% and infection to 3%. Colonization with Gram-negative enteric bacilli was highest while using hexachlorophene or Ivory bacitracin, but there was no increase in Gram-negative infections.40 Triple dye (brilliant green, proflavine hemisulphate, and crystal violet in aqueous solution) once widely used was effective against S. aureus but less effective against Gram-negative organisms.41 A 6 year study of infected umbilical stumps in the 1970s found an infection rate of 0.7% (200 of 27 107 infants) with Gram-negative organisms (n = 171) isolated more often than Gram-positive (n = 118).42
More recently, the need for umbilical cord care in Western countries has been questioned because of concerns about toxicity of topical agents, resistance and improvements in infection control which make nosocomial transmission less likely. Many institutions changed to dry cord care and non-antiseptic whole-body baths although there was no prior strong evidence for or against this change. One institution reported three cases of omphalitis following such a change (Figure 13.2).43An RCT compared antiseptic care with dry care.44 Newborns were randomly allocated to either two applications of triple dye to the umbilical cord stump on the day of birth plus twice daily alcohol swabbing until the cord fell off (n = 384) or to dry care (n = 382) which consisted of cleaning the peri-umbilical area with soap and water, wiping it with a dry cotton swab or cloth and allowing it to dry in the air. No infants in the triple dye group and one in the dry care group developed omphalitis. Infants in the dry care group were significantly more likely to be colonized with S. aureus (31.3% vs 2.8%) and with E. coli, CoNS and GBS. Community health nurses visiting infants at home were significantly more likely to report exudate and foul odour in infants in the dry care group.44 A Cochrane systematic review of umbilical cord care found 21 studies (n = 8959) mostly in high income countries.45 Antiseptics significantly reduced colonization with S. aureus by about half, but there were no systemic infections in any infants and no deaths. Umbilical infection was rare (about 1%) and not reported in most of the studies. Antiseptic cord care compared with dry cord care was associated with a non-significant but almost 50% reduction in umbilical infection (9 of 1431 vs 18 of 1400, RR 0.53, 95% CI 0.25, 1.13).45
Omphalitis is a far greater problem in developing countries and there is much stronger evidence that umbilical cord care reduces omphalitis and all-cause mortality46–48 (see Section 23.3.1). The reported incidence of omphalitis varies hugely with how cases are found and how omphalitis is defined. A hospital-based study from a special care baby unit in Oman reported an incidence of 1.8%,49 whereas a Pakistani study that used community health workers to diagnose cases found an incidence of 21.7%.50 In both countries S. aureus was the major pathogen, predominantly methicillin sensitive. In Pakistan the next most common pathogens were GAS and GBS, then Pseudomonas, Aeromonas and Klebsiella; in Oman E. coli and Klebsiella predominated.49,50
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree