Status
Disease state
I
No organic, physiologic, biochemical, or psychiatric disturbance
II
Mild to moderate systemic disturbance that may or may not be related to the reason for procedure, e.g., mild asthma, well–controlled diabetes, controlled seizure disorder, and anemia
IIIa
Severe systemic disturbance that may or may not be related to the reason for procedure, e.g., heart disease that limits activity, poorly controlled essential hypertension, diabetes mellitus with complications, chronic pulmonary disease that limits activity, and poorly controlled seizure disorder
IVb
Severe systemic disturbance that is life threatening with or without procedure, e.g., advanced cardiac, pulmonary, renal, endocrine, or hepatic dysfunction, e.g., severe bronchopulmonary dysplasia and sepsis
Vb
Moribund patient who has little chance of survival but is submitted to procedure as a last resort (resuscitative effort), e.g., septic shock, cerebral trauma, and pulmonary embolus
(b)
Airway assessment: comorbid risk factors, Mallampati classification
Factors associated with difficulty in airway management include those that make it hard to visualize the larynx or partially or completely obstruct the upper airway. Examples include: history of previous problems with anesthesia or sedation including prolonged intubation or unplanned hospitalization; stridor, snoring, or sleep apnea; chromosomal abnormality (e.g., trisomy 21); history of prematurity with prolonged intubation; significant obesity; short neck or limited neck mobility; receding mandible (small lower jaw) or decreased hyoid-mental distance; dysmorphic facial features (e.g., Pierre–Robin syndrome); small mouth opening; protruding incisors; loose teeth; dental appliances; high, arched, and narrow palate or history of cleft palate repair; large tongue; tonsillar hypertrophy; or no visible uvula (Fig. 19.1; Mallampati airway classification III, IV) [70, 71].
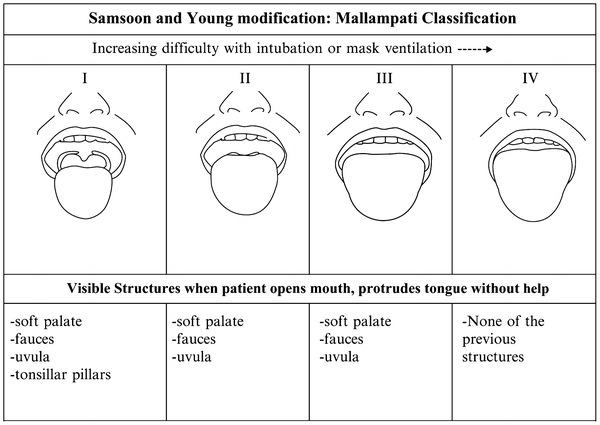

Fig. 19.1
Mallampati airway classification (adapted with permission from Mallampati SR. Recognition of the difficult airway. In: Benumof JL (ed) Airway management: principles and practice. St. Louis: Mosby-Yearbook; 1996. p. 132 [75])
Problems associated with increased risk of adverse events and for which consultation with an experienced sedation practitioner or anesthesiologist is suggested include [76]:
ASA physical status Class III or IV
Current upper respiratory illness (URI)1
Pulmonary: wheezing not cleared by a bronchodilator, obstructive sleep apnea
Morbid obesity (>2 × ideal body weight)
Cardiovascular conditions: cyanosis, congestive heart failure
Neurological conditions: poorly controlled seizures, central apnea
Gastrointestinal conditions: uncontrolled gastroesophageal reflux
Prematurity with residual pulmonary, cardiovascular, gastrointestinal, and neurological problems
Age <3 months
Pregnancy or suspected pregnancy
Neuromuscular disease
Severe developmental delay
Patients who are difficult to control
History of failed sedation, oversedation, or paradoxical response to sedatives
Screening for acute illness: Patients should be screened for acute illnesses that may increase their risk for sedation-related adverse effects. When acute illness is detected, the sedation provider must weigh the increased risk against the need for the diagnostic or therapeutic procedure.
(c)
Fasting status and risk of aspiration
To decrease the risk of pulmonary aspiration of gastric contents in healthy children undergoing general anesthesia for elective procedures, fasting from clear liquids a minimum of 2 h and from milk or solid food 6–8 h is a well-established consensus-based practice [77]. However, as noted in these guidelines, “Published evidence is silent on the relationship between fasting times, gastric volume, or gastric acidity and the risk of emesis/reflux or pulmonary aspiration in humans.” In two more recent reviews of the literature examining whether children should undergo fasting prior to ED PSA [78, 79], it is noted that little clinical data has been published to help answer this question. It is difficult to extrapolate directly to PSA from the long experience with safe general anesthesia. It is likely that risk of aspiration is less during ED PSA compared to general anesthesia in the operating room for several reasons. First, protective airway reflexes are generally preserved at the depth of moderate sedation [70, 80]. Second, airway reflexes are also relatively intact during sedation with the commonly used dissociative agent ketamine during deep sedation or even light general anesthesia [81]. Of concern, however, these reflexes are likely blunted during deep sedation with opioids, benzodiazepines, barbiturates, propofol, and etomidate, especially if sedation is deep enough to cause apnea [79]. Third, intubation of the trachea, rarely performed in children undergoing ED PSA, likely increases the risk of pulmonary aspiration due to pharmacological abolition of protective reflexes to facilitate intubation and mechanical interference with these reflexes during passage of the endotracheal tube into the trachea [73, 74, 82]. Fourth, the great majority of children receiving ED PSA meet ASA physical status Class I or II criteria [9, 61–63, 80, 83] and, compared to those in ASA physical status Classes III and IV, are associated with less risk of adverse events during anesthesia [73, 74]. It is the combination of these differences—i.e., moderate sedation, common use of dissociative ketamine for deep sedation, lack of manipulation of the larynx, and healthy patients—that likely results in ED PSA having lower risk of aspiration compared to general anesthesia.
A more robust literature on identification of risk factors for aspiration in children undergoing general anesthesia has found no benefit from routine preoperative administration of antacids or pharmacological agents to increase gastric motility [77, 84]. Gastric fluid volume or pH were not different with NPO periods of 2, 4, and 12 h after drinking apple juice in one study [85] or after 30 min to 3 h, 3–8 h, or more than 8 h after clear liquid ingestion in another trial [86]. No studies have examined gastric emptying in children after solid intake, but one small study of adult women after a light breakfast found 3 of 8 had emptied their stomachs by 2 h and all by 6 h [87].
The incidence of pulmonary aspiration during ED PSA is uncertain but appears to be very low. In a literature review of adverse events during ED PSA [78], after combining studies with a total of 4,814 children, clinically apparent aspiration during PSA was reported in only one account of two children, both of whom had fasted standard NPO periods and did not appear to be ED patients. These patients were deeply sedated with opioid–barbiturate combinations (which blunt airway reflexes), one for a radiological procedure and the other for bronchoscopy. Both required only supplemental oxygen and observation [69]. In nearly 50,000 elective propofol-based sedations, 4 children were noted to have aspirated; all recovered without sequelae after positive-pressure ventilation and supplemental oxygen and were discharged the day of or day after the procedure [88]. The incidence of aspiration in more than 100,000 children undergoing general anesthesia has been reported to be 1:978 and 1:2,632 patients by Warner [73] and Borland [74]. During emergency surgery, aspiration occurred as frequently as 1:373 patients in the Warner study [73]. Although only a rough estimate, pooling of the available data in the literature suggests the incidence of clinically apparent pulmonary aspiration during ED PSA is no more frequent than 1:2,000 pediatric patient encounters [78]. Because of the rarity of its occurrence, much larger studies are needed to accurately estimate the incidence of aspiration, and any relationship with fasting, during ED PSA. For now, given the many variables present, clinical judgment has to weigh the risk and benefits for each patient [78, 79].
Vomiting, although not likely to result in aspiration when protective airway reflexes are intact, is a common adverse event during ED PSA in children, occurring in as much as 25 % of patients, especially when opioids are coadministered prior to sedation [89, 90]. As supported by literature reviews [78, 79, 91], recent series of children receiving ketamine or nitrous oxide for ED PSA suggest there is poor correlation between the length of time of preprocedural fasting and vomiting [62, 63, 92]. No significant difference in frequency of vomiting was found between children that fasted between 0, 2, 4, 6, 8, and greater than 8 h. This may be because the vomiting is medication induced and gastric contents have little effect on likelihood of vomiting.
Gastric emptying may also be unpredictably delayed in ill or injured patients due to development of ileus [93]. ED management of pain with opioids likely exacerbates this problem. Whether brief delay (1–6 h) of PSA decreases vomiting is undetermined. Coadministration of ondansetron has been found to reduce vomiting associated with ketamine-based ED PSA but only from 12.6 to 4.7 % with 13 patients needing to be treated to prevent one episode of vomiting [94]. This and other strategies need further investigation. It is the practice of the author to consider all sedated ED patients to have “full stomachs” and to manage them with vigilance and preparation for assisting them in clearing their oropharynx by rolling them to their side or assisting them in leaning forward. Suctioning of the mouth is then used, if needed, to “mop up.”
Pregnancy: Since many medications administered for ED PSA have the potential for causing harm to a fetus, it is recommended that the menstrual status be reviewed with post-menarchal girls and a urine pregnancy test performed prior to sedation. The US Food and Drug Administration (FDA) has categorized medications based upon known or possible risk to a developing fetus as listed in Table 19.2. Increasing uterine size, greater tendency for vomiting, and many other changes also increase the complexity of PSA during pregnancy.
Table 19.2
United States FDA pharmaceutical pregnancy categories
Category A | Adequate studies have failed to demonstrate a risk to the fetus in the first trimester of pregnancy and there is no evidence of risk in later trimesters |
Category B | Animal reproduction studies have failed to demonstrate a risk to the fetus, and there are no adequate and well-controlled studies in pregnant women. Animal studies have shown an adverse effect, but adequate and well-controlled studies in pregnant women have failed to demonstrate a risk to the fetus in any trimester |
Category C | Animal reproduction studies have shown an adverse effect on the fetus and there are no adequate and well-controlled studies in humans, but potential benefits may warrant use of the drug in pregnant women despite potential risks |
Category D | There is a positive evidence of human fetal risk based on adverse reaction data from investigational or marketing experience or studies in humans, but potential benefits may warrant use of the drug in pregnant women despite potential risks |
Category X | Studies in animals or humans have demonstrated fetal abnormalities and/or there is positive evidence of human fetal risk based on adverse reaction data from investigational or marketing experience, and the risks involved in use of the drug in pregnant women clearly outweigh potential benefits |
Informed Consent
The physician responsible for the sedation should provide to the patient and/or parents’ information concerning the objectives of the sedation, behavioral changes associated with the sedative regimen (especially important when the parent/guardian plans to remain with the patient during the sedation/procedure), and potential adverse effects during and after the sedation [59, 70, 95]. Parents should understand that, albeit rare, there is a risk of pulmonary aspiration, cardiopulmonary compromise, hypoxic brain injury, and/or death. It is also recommended to discuss with them the possible need for muscle relaxation, intubation, hospitalization, and unsuccessful sedation with inability to perform the procedure. These issues that have been discussed with the parent/guardian (and patient when appropriate) and that they have given their informed consent to proceed with the sedation should be documented on the sedation record.
Adverse effects/events generally discussed include:
Incomplete analgesia and/or amnesia
Respiratory depression/apnea
Pulmonary aspiration
Psychosis and recovery dysphoria
Catatonia/nystagmus
Dysrhythmias
Plan for Sedation
(a)
Selection of a medication plan
Selection of medications and dosages should be guided by the desired key effect(s). An ideal regimen would provide acceptable analgesia, sedation, and amnesia for residual awareness of procedure-related pain or anxiety, cause minimal adverse effects, and work reliably with a wide therapeutic index; i.e., small differences in dose would not cause oversedation or adverse events, have rapid onset and recovery, and be easy to titrate to effect. No single agent or combination of agents fully achieves these goals. Selection of procedural sedation medications therefore is based upon balancing desired effects with the potential for adverse effects. For procedures that are very painful (e.g., fracture reduction), control of the pain will be paramount. For procedures that require the child to be motionless—e.g., computerized tomography (CT) or magnetic resonance imaging (MRI) scans—immobility may be most important. Most procedures in children require some combination of analgesia and immobility along with anxiolysis; therefore, sedation planning can be broadly organized into categories of these parameters.
Analgesia, hypnosis, anxiolysis, or amnesia? Balanced sedation: Medication selection and dose can be organized by anticipation of whether the procedure is (1) nonpainful/noninvasive or associated with (2) low level of pain and high anxiety or (3) high level of pain, high anxiety, or both, (4) whether local anesthesia can be used, and (5) whether the patient needs to be motionless; i.e., for some procedures, some motion is acceptable during painful and/or invasive procedures to the extent that the motion neither causes risk to the patient nor hinders the successful performance of the procedure, whereas in others (e.g., MRI), any movement prevents completing the procedure (see Table 19.3) [61, 96, 97].
Pain | Anxiety | Motion | Clinical examples | Suggestion sedation strategies |
|---|---|---|---|---|
No | Moderate | Some acceptable | Echo, EEG, infant PFTs (sedation rarely needed) | Comforting, distraction |
Chloral hydrate PO (in patients <2 years of age) | ||||
Midazolam PO | ||||
Motionless | Computed tomography | Chloral hydrate PO (in patients <6 months of age) | ||
Magnetic resonance | Pentobarbital ± midazolam IV | |||
Propofol IV | ||||
Low or local anesthesia can be used | Moderate to high | Relatively motionless | Abscess incision and drainage | Topical or local anesthesia |
But some acceptable | Dental procedures, lumbar puncture | Comforting, distraction | ||
Flexible fiber-optic laryngoscopy | Oxycodone PO | |||
Ocular irrigation | Nitrous oxide | |||
Foreign-body removal | Midazolam PO, PR, IN, IV | |||
Phlebotomy, IV cannulation | ||||
Laceration repair, simple | ||||
Fracture reduction with hematoma block | ||||
Paraphimosis reduction | ||||
Sexual-assault examination | ||||
High | Moderate to high | Relatively motionless | Abscess incision and drainage | Midazolam and fentanyl IV |
But some acceptable | Arthrocentesis | Ketamine IM or IV | ||
Bone marrow aspiration | Nitrous oxide and oxycodone PO | |||
Burn debridement | Propofol and ketamine or fentanyl IV | |||
Cardioversion | ||||
Foreign-body removal | ||||
Complicated | ||||
Fracture or dislocation reduction | ||||
Hernia reduction | ||||
Laceration repair, complex | ||||
Paracentesis | ||||
Thoracentesis | ||||
Thoracostomy-tube placement |
Principle and secondary effects of sedative–analgesic medications are summarized in Table 19.4. Although combining sedative–analgesic medications generally increases the risks of adverse effects [98, 99], the actual depth of sedation is likely to be a better predictor of these risks [96, 100]. Thoughtful “balanced sedation” with anxiolytic and analgesic drugs, carefully titrated to effect, can achieve very satisfactory sedation and typically results in smaller effective doses of individual drugs than if a single drug is used. For example, fentanyl is a potent analgesic but has little or no anxiolytic or amnestic effect, whereas midazolam is a potent anxiolytic and amnestic agent with no analgesic effect. Combining fentanyl and midazolam results in effective procedural sedation, but the combination causes significantly greater respiratory depression than either fentanyl or midazolam alone [98].
Table 19.4
Procedural sedation medication effects
Medication | Sedation | Analgesia | Amnesia | Anxiolysis | Emetogenic |
|---|---|---|---|---|---|
Barbiturates | +++ | − | − | − | |
Benzodiazepines | +++ | − | +++ | +++ | Antiemetogenic |
Fentanyl | + | +++ | − | ++ | |
Ketamine | +++ | +++ | ++ | + | |
Propofol | +++ | − | + | + | Antiemetogenic |
Chloral hydrate | ++ | − | – | ||
Nitrous oxide | ++ | ++ | + − ++ | +++ | ++ |
Depth of sedation: Since increasing depth of sedation is associated with increasing frequency of adverse events [96, 101], use of the lightest effective sedation is usually preferred. However, frequently the depth of sedation required for a particular procedure cannot be accurately predicted in a specific patient [96]. Incompletely appreciated anxiety and lack of comprehension in younger children or those with developmental delay often cause need for deeper-than-anticipated sedation for procedures in which local anesthesia or mild sedation would suffice in a self-controlled adolescent or adult. For intensely painful procedures, deep sedation is typically required. Clinicians providing sedation, therefore, ideally should be trained and prepared to administer increasingly deeper sedation as guided by the patient’s response to the procedure. It is important, too, for the clinician to realize that many sedative analgesic agents also induce varying degrees of amnesia. When midazolam, ketamine, or propofol, and to a lesser extent nitrous oxide, are administered, the patient is unlikely to recall clearly procedure-related pain despite occasional moaning or crying out during intensely painful parts of the procedure [9]. However, it is unwise to promise complete amnesia during the informed consent process. The extent of procedural amnesia can be assessed in part by asking the patient if he/she “recalls anything hurting” after they have recovered; a negative answer is reassuring to parents who have remained with the patient during the procedure. Because of amnesia for procedure-related pain, lighter and presumably safer levels of sedation may be acceptable when patient motion does not interfere with accomplishment of the procedure.
For this reason, the amnestic agent midazolam is combined with fentanyl for PSA because completely effective analgesia cannot be achieved with fentanyl without marked respiratory depression. Of note, deeper sedation with ketamine is usually much less associated with adverse cardiopulmonary effects in comparison to other agents and, in addition, ketamine induces moderate amnesia.Some older children may prefer not to be deeply sedated; in the same way many adults fear general anesthesia. As an example, a 13-year-old boy sedated by the author with nitrous oxide in conjunction with a lidocaine fracture hematoma block recalled the next day the details of the reduction of his displaced distal radius and ulnar fractures. Yet, he was adamant that he would not have preferred to have been “put to sleep” and unaware of the reduction. Since the hematoma block was very effective and he recalled no pain, he was very satisfied with his experience of altered awareness during the fracture reduction. When local anesthesia or other analgesic technique can be achieved, some children may prefer lighter levels of sedation without loss of awareness, a concept that needs further investigation.
(b)
Staffing
For moderate sedation, a sedation provider trained in the sedation protocol and skilled in pediatric advanced life-support techniques is responsible for the procedural sedation–analgesia, including monitoring of the patient’s status. In the ED, this is typically the emergency physician. If, after induction of adequate sedation, that individual then performs the procedure for which sedation is provided, a second individual, typically a registered nurse, with sedation training and knowledgeable in pediatric basic life support must be at the bedside and responsible for monitoring the patient’s cardiopulmonary status and the need for interventions to manage adverse events. This second individual often is responsible for recording the patient’s status on the sedation record and may assist with minor, interruptible tasks once the patient’s level of sedation and cardiopulmonary functions have stabilized, provided that adequate monitoring of the patient is maintained [59, 68, 70, 102].
For deep sedation in the ED, a sedation provider, again, typically the emergency physician, with training in the pharmacology of the agents to be administered and skilled in pediatric advanced life support must be in the procedure room and is responsible for the procedural sedation–analgesia, including monitoring of the patient’s status. At least one clinician must be assigned to monitor and record the patient’s airway patency and cardiorespiratory status and, in contrast to moderate sedation planning, should have no other responsibilities during induction of sedation, the procedure, and the early postprocedure period when the patient is at greatest risk for respiratory depression, partial upper airway obstruction, and aspiration. If an experienced sedation provider has induced adequate sedation and will then perform the procedure, primary responsibility for monitoring the patient’s cardiopulmonary status may be designated to a second sedation-trained clinician, typically a registered nurse, if the responsible provider can easily interrupt performance of the procedure to assist with or assume management of adverse events. It should not be planned that the clinician monitoring the patient would assist with the procedure as that may distract this clinician from monitoring the patient’s vital signs and clinical status or interfere with rapid intervention [59, 68, 70, 102, 103]. Brief, interruptible assistance with the procedure may be provided by this person but with caution and with assured concurrent attention to the patient’s vital functions. Safe use of deep sedation is dependent upon this clinician’s meticulous attention to the patient’s airway and breathing and anticipation and early recognition of adverse events. Threats to ventilation and oxygenation usually are easily managed when rapidly recognized and interventions immediately implemented. Experience with deep sedation has shown that some patients (~5–25 %) will develop oxygen desaturation of <90 % and partial upper airway obstruction, both of which are usually easily managed when rapidly recognized.
Since deeper-than-intended sedation may occur or be necessary in any patient, it is recommended that all but the lightest sedations (e.g., use of nitrous oxide) be staffed and monitored as if deep sedation may occur, particularly when gaining initial experience with sedation protocols or using agents with narrow therapeutic indices (e.g., propofol, midazolam + fentanyl, or etomidate). This usually means a third provider is needed if assistance will be necessary in performing the procedure. In addition, at least one provider should be present who is intimately familiar with location of resuscitation and other necessary medical equipment.
In most hospitals, physician sedation providers and nurses must be credentialed to administer PSA. Credentialing typically includes didactic sessions on use of specific PSA medications, demonstration of safe and effective administration of PSA, and competency in skills needed for rescue from adverse events [95].
(c)
Monitoring and equipment
Direct patient observation: In addition to electrophysiological monitoring, airway patency, rate and depth of respiration, and the child’s color (nail beds, mucosa) should be checked frequently by vigilant direct observation, especially after each medication administration and in the early postprocedure period when painful procedural stimuli have ended. This enables essential immediate interventions for adverse events such as marked respiratory depression, positional obstruction of the upper airway as muscle relaxation occurs (snoring, paradoxical chest wall motion without exhaled breaths may be noted), or vomiting. Opening of the airway by realignment or jaw thrust, applying painful stimulation to awaken and induce breathing, administering supplemental oxygen, or turning and suctioning to clear vomit often are usually all that is needed to correct problems that can otherwise rapidly deteriorate to life-threatening situations.
Direct monitoring during recovery should continue by a designated healthcare provider until the patient emerges to a level of moderate sedation; thereafter direct monitoring can be designated to the child’s parent or another responsible adult with the healthcare provider immediately available until the patient returns to the pre-sedation level of responsiveness [59, 68, 102, 103].
Patients undergoing sedation should wear a loose-fitting top or hospital gown to ensure easy direct observation of the chest. The patient’s mouth and nose should not be obscured and skin should be visible for monitoring of color. A stethoscope should be immediately available.
For moderate sedation, in addition to direct observation, measurement of oxygen saturation by pulse oximetry is strongly recommended [59, 68, 102, 103]. Additional continuous electrophysiological monitoring throughout sedation and recovery of ECG-based heart rates, respiratory rates, and noninvasive automated blood pressures measured after each medication bolus and/or every 5 min add further measures of safety.
For deep sedation, in addition to direct observation, routine use of noninvasive physiologic monitoring should include continuously measured oxygen saturation, heart rate, and respiratory rate, and, in addition, noninvasive automated blood pressure measurements after each medication bolus and/or every 5 min throughout sedation and recovery [59, 68, 102, 103].
Pulse oximetry has been demonstrated to detect hypoxemia well before cyanosis occurs and is therefore critical for monitoring for respiratory compromise. In one study of infants, O2 saturations were ≤83 % before perioral cyanosis was detected by experienced emergency pediatricians [104]. Monitoring of oxygen saturation with pulse oximetry has been suggested as the most important means of reducing sedation-related injury and should be used for all but minimal sedations [59, 68, 70, 100, 102, 103]. The pulse oximeter audible tone should be activated to alert providers to changes without the need to frequently read the monitor instead of observing the patient.
End–tidal CO 2 capnography provides breath-to-breath information on the effectiveness of ventilation and is increasingly being investigated in patients undergoing ED PSA. Assessment of ventilation by continuous end-tidal CO2 capnography has been found more sensitive than either direct observation or decreases in oxygen saturation in detecting respiratory depression or airway obstruction. Changes in capnographic waveform and/or changes in end-tidal CO2 are frequently noted well before changes in oxygen saturation, including in patients’ breathing room air [105–111]. Of note, no changes in end-tidal CO2 were found in children sedated with ketamine alone [112, 113]. Changes in end-tidal CO2 capnography can aid in early recognition of respiratory depression and/or airway obstruction and allow initial interventions that may avert the need to administer positive-pressure ventilations, e.g., limitation of further administration of sedative medications or opening of the airway. Assisted ventilation during oxygen desaturation due to apnea or periods of respiratory depression should be administered as needed. However, positive-pressure ventilation increases gastric pressures due to insufflation of air into the stomach. At a depth of sedation that induces apnea or significant respiratory depression, there is likely concurrent relaxation of esophageal muscle tone and significant blunting of protective airway reflexes. Thus, there is likely increased risk of pulmonary aspiration associated with positive-pressure ventilation due to gastroesophageal reflux into the oropharynx.
Routine administration of supplemental oxygen has been recommended to prevent hypoxemia during deep and moderate sedation [103]. However, sedation providers should recognize that administration of supplemental oxygen may delay oxygen desaturation for several minutes during respiratory depression or apnea [114]. Therefore, use of supplemental oxygen may delay recognition of these adverse events with their likely concurrent depression of protective airway reflexes, unless the patient is also monitored by end-tidal CO2 with capnography [115]. Similarly, recognition of airway obstruction is likely delayed [105–109, 112, 116]. When capnography is unavailable, consideration should be given to monitoring patients by pulse oximetry as they breathe room air. Although an indirect and less-sensitive measure of ventilation than capnography, decreases in oxygen saturation alert the clinician to decreases in ventilation and facilitate interventions before hypoxemia and a need for positive-pressure ventilation occurs. With this strategy, administration of supplemental oxygen may be reserved for patients whose oxygen saturations drop below 90 % without rapid rise in response to airway maneuvers such as head tilt/jaw thrust and/or stimulation. Respiratory depression is sufficiently commonplace during sedation with propofol that many providers recommend as routine administration of supplemental oxygen during propofol PSA [107, 108, 117].
Equipment
Resuscitation equipment must be immediately available. A self-inflating (Ambu-type) bag–mask positive-pressure device with a PEEP attachment and appropriately sized mask, continuous oxygen supply, and an airway suctioning device with a large rigid suction tip should be prepared for each sedation. Anesthesia-style CPAP bags, endotracheal intubation equipment, and resuscitation medications, with a dosing guide, including reversal agents such as naloxone and flumazenil, a paralytic agent such as succinylcholine, and antiepileptic and antiarrhythmic medications for drug-induced seizures and dysrhythmias should be immediately available for all sedations [59, 68, 70, 102, 103].
No suction apparatus can clear the oropharynx during active vomiting. The patient must be helped to turn or roll to the side or to sit upright to clear his airway. The suction device is used to clear residual emesis from the mouth after active vomiting has stopped. If the patient is unresponsive and emesis is noticed in the posterior pharynx or mouth, the patient should be rapidly rolled to the side to allow emesis to passively flow out as suctioning of the posterior pharynx is performed; there is significant risk for pulmonary aspiration in this situation.
Intravenous access adds an additional invasive procedure to the patient’s treatment, but it enables easily controlled and rapid titration of medications and provides an increased margin of safety by enabling rapid administration of reversal and resuscitation agents, if needed. When medications are administered intravenously, the intravenous access should be maintained throughout sedation and recovery. When medications are administered by a non-intravenous route (e.g., by intramuscular injection), whether to establish intravenous access should be decided on an individual basis. If vascular access is not established, the ability to immediately accomplish such must exist for all sedations, especially when a multiple drug sedation regimen is used. For agents that frequently cause hypotension (e.g., propofol), it is recommended that intravenous access be established with an indwelling catheter and maintained with a resuscitation fluid (lactated Ringer’s solution or normal saline). Patients who have been NPO for an extended period may benefit from an infusion of 10–20 mL/kg of LR or NS to counter any hypotensive effects of sedation medications. A stopcock near the hub of the IV catheter (e.g., on the tail of a T-connector inserted into the hub of the catheter and in-line with the IV fluids) facilitates controlled and complete administration of sedation medications. This setup allows a syringe containing the sedative to be connected to the stopcock and the medication injected near the hub as the IV fluids infuse. This reduces the possibility of uncertain medication infusion amount and rate that might occur if the medication is added considerably upstream of the catheter hub. For agents such as ketamine that do not frequently cause hypotension, an indwelling “saline lock” is typically sufficient; the ketamine can be flushed into the bloodstream with 5–10 mL boluses of saline following ketamine administration.
A mnemonic some find helpful in preparing equipment is MS MAID: Machine Suction – Monitors Airway (oral airway, bag-mask, ETT, blade) IV Drugs.
Preparation for and Management of Adverse Events
Anticipation
The rarity of serious adverse events in ED PSA can lull the provider into complacency [118, 119]. It is suggested that the possibility of a life-threatening event during PSA should be thought of as inevitable, as a matter of “when” rather than “if.” Since these events are so infrequent and variations in individuals’ responses to a medication are not always predictable, the provider must always be prepared.
Effective management of adverse events begins first and foremost with preparation for the planned sedation. Thorough pre-sedation evaluation to identify patients at increased risk for adverse events or difficult airway management, monitoring and staffing based upon intended sedation depth, and immediate availability of resuscitation equipment and medications are critical. Factors associated with serious adverse outcomes include late recognition of hypoxemia and inadequate resuscitation, thus emphasizing the importance of preparation and continual monitoring during the sedation and recovery periods [100]. If recognized early, most adverse effects can be addressed effectively with relatively minor interventions. Stimulation, airway realignment, jaw thrust, and supplemental oxygen are usually all that is needed to avoid further deterioration to life-threatening events [119].
Management of Respiratory Depression and Apnea
Respiratory depression is one of the most common potentially serious effects of pediatric PSA [67, 118, 119]. A critical incident analysis of serious adverse outcomes in pediatric sedation found 80 % initially presented with respiratory depression [100]. Widespread use of pulse oximetry has since dramatically improved early recognition of respiratory depression. Agents commonly associated with respiratory depression include the sedative–hypnotics (barbiturates, benzodiazepines, chloral hydrate, propofol), particularly when used in conjunction with opioids [101, 120]. Apnea has also been rarely reported with administration of ketamine [121–123].
Avoiding respiratory depression (see also Basic Pharmaco-kinetics): Most sedative medications variably blunt brainstem receptor response to increases in plasma levels of CO2. Since response to rising levels of CO2 determines breathing rate and depth, significant increases in sedative concentrations in the brainstem quickly lead to respiratory depression or apnea. The more rapidly a sedative drug is infused, the higher its initial brainstem concentration and the greater the respiratory depression. A primary strategy for reducing respiratory depression and maintaining adequate ventilation (and, in association, oxygenation) is slow administration of PSA drugs, often achieved by repeatedly infusing half or less of the total expected dose until the desired effect is achieved (titration). Ketamine can be an exception to the recommended slow administration approach because of its unique relative lack of respiratory depression. Taking advantage of first-pass kinetics, experienced sedators may choose to administer smaller doses rapidly for very brief procedures (see “Ketamine” section).
At-risk periods: Patients may experience respiratory depression at any time during the sedation, but the greatest risks are immediately after medication administration and again after cessation of painful procedural stimuli [124].
Recognition of ineffective ventilation: As detailed previously, direction observation of the patient including general color and chest wall movement continues to be one of the most important means of recognizing respiratory depression and/or airway obstruction. The patient’s oropharynx and chest wall should be directly visible at all times to facilitate observation for lack of respiratory effort or respiratory effort without air exchange. In addition, pulse oximetry with audible tone and end–tidal capnography facilitate detection of ventilatory changes before they are clinically apparent.
Airway and Ventilation Maintenance
Initial management of hypoventilation may simply require verbal encouragement to the patient to breathe as their sensitivity to rising CO2 has been blunted by the sedation medications. Patients who have received opioids such as fentanyl may be awake but “forget” to breathe. Stimulation, painful if necessary, to arouse the patient may improve muscle tone and prompt breathing. If oxygen saturations are falling despite these maneuvers, supplemental oxygen administration and airway-opening maneuvers and/or positive-pressure ventilation may be necessary. See section below for management of “Upper Airway Obstruction.”
Treatment: Respiratory Depression and Apnea
When monitors alarm (e.g., indicating dropping oxygen saturation), ASSESS THE PATIENT. DO NOT presume the pulse oximeter probe has slipped off, monitor malfunction, etc. Evaluate equipment later!
First Line (in Rapid Succession, if Needed)
1.
Verbally encourage or stimulate patient to breathe deeply (patients may require intensely painful stimuli, e.g., squeezing the fracture site or a hard sternal rub with knuckles); if insufficient, then do number 2.
2.
Support airway (chin lift/jaw thrust); if insufficient, then do number 3.
3.
Administer supplemental oxygen.
4.
If spontaneous ventilation continues to be inadequate, administer positive-pressure ventilation via bag–mask.
5.
If patient is on a continuous infusion (e.g., propofol)—slow down or stop medication infusion, and then do number 6.
6.
Call for help, if needed.
Second Line: Reversal Medications for Opioids and Benzodiazepines
If respiratory depression occurs after administration of an opioid or benzodiazepine and does not readily resolve after the above supportive measures, or requires continued positive-pressure ventilation, consider use of reversal agents. Slow, titrated reversal is preferred if positive-pressure ventilation is effective. The desired endpoint is lessening of the respiratory depression with slightly lighter sedation. Rapid, full reversal may lead to severe pain, hypertension, and agitation or seizure [125]. Reversal agents are rarely needed by experienced sedation providers.
Naloxone (Narcan®)
Indications: opioid-induced apnea, respiratory depression, or “wooden/rigid chest syndrome” not responding to stimulation, airway-opening maneuvers, supplemental oxygen, and/or positive-pressure ventilations.
Dose: 1–2 mg/kg (0.001–0.002 mg/kg) IV push repeated every 1–3 min until the patient begins to have spontaneous respirations. Doses of 1–2 mg/kg are recommended to “gently” reverse opioid-induced respiratory depression yet maintain analgesia. Larger doses, such as 10–100 mg/kg, may awaken the patient and reverse the analgesic effects resulting in significant pain, hypertension, pulmonary edema, vomiting, or seizures [125].
During the interval of apnea, the patient is supported with assisted ventilations until adequate spontaneous respirations are restored. Thereafter, the patient is observed closely as the reversal effects of naloxone may be briefer than the opioid-induced respiratory depression. For “wooden chest syndrome,” if the patient cannot be ventilated and oxygen saturations are dropping rapidly, naloxone may be given in 1 or 2 mg boluses for convenience. Alternatively, succinylcholine 1–2 mg/kg may be used to paralyze the patient.
Caution: Opioid-induced respiratory effects may outlast the duration of naloxone, and patients must be closely monitored for recurrence of respiratory depression, typically at least 2 h after naloxone administration [126].
Flumazenil (Romazicon®)
Indications: Benzodiazepine (e.g., midazolam)-induced apnea or respiratory depression not responding to stimulation, airway-opening maneuvers, supplemental oxygen, and/or positive-pressure ventilation.
Dose: 0.01–0.04 mg/kg (maximum 0.5 mg) IV over 30 s. Repeat every 60 s to desired response. A cumulative dose of 3 mg may be necessary. Flumazenil may reverse midazolam-induced hypnotic and amnestic effects but may not reverse ventilatory depression [127]. When appropriate, naloxone should be used as the first line in reversal therapy. Drug therapy does not obviate the need to protect the airway and support ventilation.
Caution: Flumazenil may cause seizures in patients chronically on benzodiazepine medications and should be used cautiously in patients on medications that can lower seizure threshold. Also, benzodiazepine-induced respiratory effects may outlast the duration of flumazenil, and patients must be closely monitored for recurrence of respiratory depression, typically at least 2 h after flumazenil administration [128, 129]. Recurrence of sedation has been reported in up to 7 % of cases, most commonly in children under 5 years of age [128] (Table 19.5).
Agent | Route | Dose | Frequency | Maximum dose (mg) | Onset | Duration (min) |
|---|---|---|---|---|---|---|
Naloxone | IV, IM, or SC | 1–2 μg/kg for respiratory depression | Q 2–3 min as needed | 2 | 1–2 min (IV) | 30–60 |
100 μg/kg (0.1 mg/kg) if unable to ventilate or wooden chest | 15 min (IM/SC) | |||||
Flumazenil | IV | 10 μg/kg (0.01 mg/kg) | Q 1 min as needed | 1a | 1–2 min, maximum effect 6–10 min | 20–60 |
Upper Airway Obstruction
The pediatric airway is particularly prone to dynamic obstruction due to the relatively large size of the tongue and tonsillar tissues. As sedation depth increases, the muscles of the tongue, jaw, and oropharynx lose tone in a manner similar to deep sleep. Sedation-induced “obstructive sleep apnea” may result in partial or complete airway obstruction, exacerbated by the supine position and nasal passage obstruction. A history of snoring or obstructive sleep apnea alerts the clinician to the increased likelihood of this occurrence. Placement of a shoulder roll in infants and a head roll in older children and adolescents to align the oropharynx, posterior pharynx, and trachea may help align the patient’s airway and relieve this obstruction. Markedly, obese patients also may benefit from a large head or shoulder roll to compensate for their large trunk.
A jaw thrust or chin lift may be necessary to open the upper airway by pulling the tongue and related muscles away from the posterior pharynx. Patients who are very deeply sedated or have inadvertently reached the depth of general anesthesia may benefit from placement of an oro- or nasopharyngeal airway, but because oropharyngeal airways may induce a gag reflex and vomiting, these devices should be used with caution. Laryngospasm is a special type of upper airway obstruction and is addressed later in this chapter.
At-risk periods: Positional airway obstruction may occur at any time during sedation but, in association with respiratory depression, it may more likely be shortly after medication administration or after the painful procedural stimulus has ended. Ketamine-related laryngospasm may occur in settings of current URI, unsuctioned secretions/vomitus, or stimulation of the hyperactive gag reflex during a procedure.
Recognition of upper airway obstruction: Signs of partial upper airway obstruction include stridor or noisy breathing. Paradoxical chest wall movement (sucking in of the chest and distention of the abdomen with inspiration) may be seen with partial or complete obstruction. Hypoxemia is a late sign. An obstructive pattern is seen on capnography well before changes in oxygen saturation and allows early detection of airway obstruction (or apnea).
Treatment
1.
Align airway and open with chin lift or jaw thrust; provide supplemental oxygen as needed.
2.
Suction airway if excessive secretions are present.
3.
If not responding to repositioning, consider continuous positive airway pressure (CPAP) with bag–mask (CPAP or anesthesia-type bag is preferable to self-inflating-type bag as CPAP can be delivered more effectively to open the airway by distending the posterior pharynx).
4.
If having difficulty maintaining an open airway, consider an oral airway (unconscious patient) or nasal airway.
5.
If unable to ventilate with CPAP, rapidly consider treatment for laryngospasm with succinylcholine.
Laryngospasm
Laryngospasm is an uncommon but potentially life-threatening sedation-related adverse event. It is a partial or complete upper airway obstruction, with oxygen desaturation, caused by involuntary and sustained closure of the vocal cords and is not relieved by routine airway repositioning maneuvers, suctioning, or insertion of a nasal or oral airway. Laryngospasm may be intermittent or sustained and brief or prolonged [130, 131].
The incidence of laryngospasm during pediatric ED PSA is difficult to determine as it is a rare event and large sedation databases are not available for estimation. Relative preservation of upper airway protective reflexes during ketamine-based sedation reduces the risk of pulmonary aspiration and thus makes ketamine one of the safest agents for ED PSA in unfasted children, yet, paradoxically, ketamine PSA may have increased risk for laryngospasm [132–134]. A meta-analysis of pediatric ketamine-based ED PSA found an incidence of laryngospasm of 0.3 %; the only identifiable association with increased risk of laryngospasm was an initial intravenous dose of greater than 2.5 mg/kg, but data was unable to be analyzed for associations with URI, wheezing, or other risk factors found to be associated with increased risk during general anesthesia [135]. Of particular interest, young age and oropharyngeal procedures (excluding endoscopy) were not associated with increased risk, but prospective larger data sets are needed to better clarify these risks.
Laryngospasm in almost 50,000 non-intubated children undergoing elective propofol sedation/anesthesia was noted to occur at a rate of 21/10,000 (0.2 %) [88]. Laryngospasm associated with general anesthesia has been estimated as high as 14 % in younger children and as low as 0.1 %, with lower likelihood reported in non-intubated children [136, 137]. The wide variability may be due to differences in definition and study design, patient populations, anesthetic techniques, and airway manipulation [138]. However, consistently noted risk factors for laryngospasm include young age, upper respiratory infection, asthma, manipulation of the airway, and exposure to smoking in the home [139, 140].
It is unclear whether prophylactic administration of atropine or glycopyrrolate with ketamine to reduce hypersalivation reduces the risk of laryngospasm [141, 142]. The meta-analysis of pediatric ketamine-based ED PSA, noted earlier, found that overall airway and respiratory adverse events (but not laryngospasm) were actually increased in children who received concurrent anticholinergics [135]; this unexpected association needs further investigation.
At-risk periods: Laryngospasm may occur at any time during sedation, including recovery. In one report of non-intubated children undergoing sedation/general anesthesia, laryngospasm occurred most frequently during emergence (48 %) but was also seen during induction (29 %) and maintenance (24 %) phases [137]. Increased risk for ketamine-related laryngospasm may occur in children with current URI, especially if febrile, if secretions/emeses pool in the posterior pharynx, or if a procedure such as endoscopy stimulates the gag reflex [140, 143, 144].
Recognition of laryngospasm: Early signs of laryngospasm may include coughing. A characteristic stridulous noise can be heard with partial laryngospasm. Chest wall movement is noted, but there is a mismatch between the patients’ respiratory effort and the small amount of air exchange. If complete laryngospasm occurs, no stridulous noise will be heard and no air exchange or breath sounds will be noted despite chest wall movement. No ventilation with a bag–mask device will be possible.
Oxygen saturations will drop rapidly if the patient is breathing room air, typically within 30–60 s. If the patient has been preoxygenated, saturations may remain above 90 % for 1–5+ min, dropping more rapidly in younger children and infants [114]. Capnographic changes are a very sensitive means of diagnosing laryngospasm. During partial laryngospasm, turbulence affects expiratory flow, but the amplitude of the capnogram will correlate with the extent of hypoventilation. During complete laryngospasm the CO2 waveform will be lost despite chest wall movement [110].
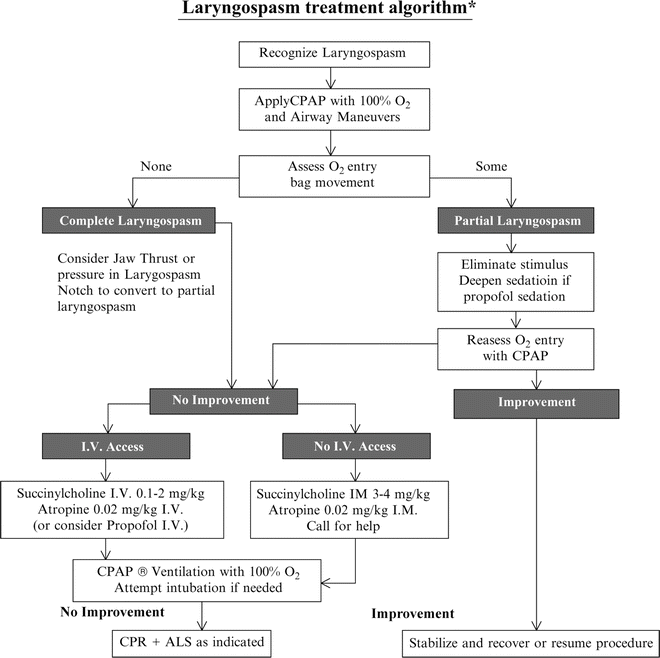
Treatment (Fig. 19.2) [134]
If the patient develops stridor during sedation:
1.
Remove stimulus to posterior oropharynx; consider gentle suction of excessive secretions and emesis.
2.
Reposition airway with jaw thrust; vigorous, painful intrusion of the thumbs in the laryngospasm notch 2 may help.
3.
Apply CPAP (continuous positive airway pressure) with 100 % O 2 with anesthesia-type bag–mask; CPAP may reduce partial obstruction by distending the posterior pharynx, which exerts pull to open the partially closed larynx and vocal cords.
4.
Assess air movement, if unable to oxygenate with CPAP.
5.
Rapidly consider atropine 0.02 mg/kg IV followed by low-dose succinylcholine(0.1–0.25 mg/kg IV) with ventilatory support as needed [146]; consider an additional dose of propofol if propofol sedation is underway.
6.
If still unable to oxygenate, administer full–dose succinylcholine (1–2 mg/kg IV or 3–4 mg/kg IM) followed by intubation.
Attempts to provide intermittent positive-pressure ventilation with a face mask may distend the stomach and make subsequent ventilation more difficult. In complete laryngospasm CPAP may worsen the obstruction by forcing the area just above the false cords closed. Therefore, if complete spasm cannot be broken, early IV agents should be considered [134].
When laryngospasm occurs in the midst of propofol PSA, deepening the sedation with administration of an additional 0.5 mg/kg of propofol has been shown to be an effective treatment for laryngospasm [147]. Transient apnea with this technique should be anticipated.
Low–dose succinylcholine (0.1 mg/kg IV) may be effective in relaxing laryngospasm [146]. Onset of neuromuscular blockade is generally more rapid at the larynx compared with the peripheral muscles [148]. Relaxation of the larynx induced with this small dose will be brief but may allow the patient to be oxygenated by CPAP and intubation avoided. Alternatively, administration of a fully paralyzing dose (1–3 mg/kg IV) followed by intubation should be considered if the patient is rapidly becoming severely hypoxic [134]. The intravenous route is preferred for administration of succinylcholine, but if there is no vascular access, it can be administered intramuscularly at a dose of 3–4 mg/kg. Although full effect may take about 4 min, onset of relaxation of the larynx occurs earlier than maximum suppression of the muscle twitch response and enables ventilation [149].
Succinylcholine administration following hypoxia may be associated with severe bradycardia and even cardiac arrest. Atropine 0.02 mg/kg IV administered prior to succinylcholine is recommended [150].
Emesis
Nausea and vomiting occur in 5–25 % of children during or after ED PSA. Use of opioids before or during sedation increases the likelihood of vomiting [90, 151], whereas concurrent use of midazolam with an opioid [9], ketamine [89], or nitrous oxide [10] reduces the incidence of PSA-related vomiting. Propofol appears to be less emetogenic and may not benefit from addition of midazolam to the regimen. Coadministration of ondansetron (Zofran®) with ketamine reduces vomiting both in the ED and after discharge [94]. Children with a history of prior postoperative nausea and vomiting or with a history of motion sickness are at increased risk for vomiting [152]. Further investigations are needed to better predict sedation-associated nausea and vomiting and to determine strategies to significantly reduce this relatively minor but very undesirable adverse effect.
At-risk periods: Emesis may occur at any point during procedural sedation but most commonly is seen during the postprocedure recovery period [9, 10, 90]. Since emesis can occur at any point and with every systemic agent used for procedural sedation, the provider responsible for monitoring the patient’s airway should always be vigilant for signs of impending retching and prepared to turn the patient to the side to clear the airway. Suction equipment should be prepared and immediately available during and after all sedations. This equipment is used to finish clearing the emesis from the mouth after the patient stops vomiting. It is also advisable to have a large emesis basin at the bedside during each ED PSA.
Treatment: Emesis During Procedural Sedation
Position patient’s head to side, allow patient to clear own mouth during active vomiting, and suction oropharynx with rigid large bore Yankauer-type suction tip.
If using nitrous oxide, immediately remove the mask to allow clearing of emesis and discontinue nitrous use, at least temporarily. It is preferred to allow the patient to hold the face mask during sedation with nitrous oxide so that they can immediately remove the mask if they feel nauseated.
Ondansetron (Zofran®)
An anti-serotonin agent, ondansetron is not routinely administered to prevent emesis during ED PSA. However, one study of children receiving ketamine for ED PSA showed that vomiting in the ED or after discharge was less frequent with ondansetron coadministration: 8 % versus 19 %, with 9 patients needing to be treated to prevent one episode of vomiting [94]. Ondansetron also may be considered in a child with significant prior history of postoperative nausea and vomiting. Further evaluation of the effectiveness of this antiemetic agent during ED PSA is needed. Other antiemetic agents such as prochlorperazine (Compazine®) and promethazine (Phenergan®) usually are not used because of sedating effects and increased risk for causing dystonic reactions.
Dose: IV, PO; 0.1–0.15 mg/kg, maximum dose 4 mg. Rapidly dissolving 4 mg oral tabs (ODT) are available and can be split in half for easy administration to young children. Dosing can be simplified by administering ondansetron ODT 2 mg to children 3 years of age and younger and 4 mg to children 4 years of age and older.
Cautions: may rarely cause bronchospasm, tachycardia, headaches, and lightheadedness.
Not requiring patients to drink fluids prior to discharge also may reduce vomiting. Historically, assuring patients can drink prior to discharge has been done to prevent postoperative “dehydration.” Given shortened fasting times and the common practice of administration of IV fluids during sedation, the risk of dehydration is low compared to the risk of inducing vomiting [151].
Pulmonary Aspiration
Clinically significant or life-threatening pulmonary aspiration of gastric contents during pediatric procedural sedation is extremely rare. Aspiration occurs in approximately 0.1 % of cases under general anesthesia and was noted to have occurred in 4 of 49,836 children undergoing elective propofol sedation/anesthesia, but it has not been reported in association with ED PSA [73, 74, 80, 88]. Patients with ASA physical status Class III or higher and those requiring intubation are likely at higher risk. Risk for aspiration is likely greater, too, in patients who experience brief periods of apnea or significant respiratory depression as esophageal tone and protective airway reflexes may be absent during these periods and gastric contents may reflux into the trachea with little or no initial patient response. Because of the potential gravity of this adverse event, it is suggested clinicians consider using ketamine or nitrous oxide that better preserves protective airway reflexes or, when possible, lighter sedation combined with local anesthesia for non-fasted emergency patients [153].
Recognition: Clinical symptoms of pulmonary aspiration may include cough, crackles/rales, decreased breath sounds, tachypnea, wheeze, rhonchi, and respiratory distress that were not present before the sedation and present before the end of the ED recovery phase. These are usually accompanied by a decrease in oxygen saturation from baseline, requiring supplemental oxygen, and, if obtained, focal infiltrate, consolidation, or atelectasis on chest radiograph [80, 130]. As noted previously, clinically significant pulmonary aspiration may more likely occur in the unresponsive patient when gastric contents passively flow out of the stomach to the larynx. As the aspiration occurs, there may be little or no immediate signs due to the depth of sedation/anesthesia. The aspiration may become evident as the patient emerges from sedation.
Treatment: If emesis is seen, turn patient to side, allow to retch, and suction posterior pharynx as needed. Administer supplemental oxygen by nasal cannula or mask as needed. Many cases of transient hypoxia will resolve with this simple maneuver. CPAP may improve oxygenation in cases of severe aspiration with alveolar collapse. The majority of children who experience pulmonary aspiration require only close observation and simple supportive care with supplemental oxygen with or without CPAP and recover without sequelae [73, 74, 84, 88]. Endotracheal intubation should be considered if definitive protection of the airway or tracheal suctioning is required; RSI (rapid sequence induction) may be necessary. Uncommonly, severely symptomatic patients may need to be taken to the OR for emergent bronchoscopy with bronchial lavage of particulate matter. Arrange for appropriate continued monitoring, support, and work-up as needed including chest radiograph. For symptomatic patients, this usually means inpatient admission to an intensive care unit.
Medications
Basic Pharmacokinetics: Simplified
Parenteral drugs effective for PSA are small, hydrophobic lipophilic compounds that rapidly diffuse out of the bloodstream into the lipophilic tissues of the brain and spinal cord where they cause sedation/anesthesia.
Since the brain receives a disproportionately high percentage of the cardiac output (15–25 %) [154], a large portion of a sedative drug injected into the bloodstream circulates on first-pass out of the heart into the brain’s circulation and quickly crosses the blood–brain barrier to exert its clinical effects within a single circulation time (first-pass or “one arm–brain” kinetics). As the drug circulates throughout the body and diffuses into muscle, bone, and, at a slower rate, into poorly perfused fat, the blood plasma concentration falls. The concentration gradient between the brain and the blood then favors drug diffusion out of the brain. As the brain’s drug concentration falls, the drug effect lessens. This secondary reequilibration (“biphasic redistribution”) causes the patient to awaken or respiratory depression to lessen. These effects are relatively independent of metabolic clearance of the drug from the body. PSA drugs’ metabolic half-lives tend to be on the order of hours, whereas their sedative effect half-lives or “wake-up times” are on the order of minutes [155].
The duration of action of a single intravenous dose is similar for all these anesthetic/hypnotic drugs and is determined by redistribution of the drugs out of the brain. However, after repeated doses or prolonged infusions, a drug’s duration of action is determined by complex interactions between the rate of redistribution of the drug, the amount of drug accumulated in fat, and the drug’s metabolic clearance. The wake-up time of some drugs such as etomidate, propofol, and ketamine increases only modestly with prolonged infusions while others such as diazepam and thiopental increase dramatically and midazolam less so [155].
A rapidly injected drug travels as a more concentrated bolus on the first-pass out of the heart into the brain circulation than a slowly injected drug that is diluted by the passing blood. Thus, with rapid infusion, the initial concentration gradient between the plasma and the brain is greater. Consequently, the brain’s concentration of the drug rises more rapidly and a greater portion of the administered dose enters the brain with resultant deeper sedation than when the same drug dose is slowly infused.
Thus, small doses of medications can have significant clinical effect if administered rapidly. Since the blood–brain concentration gradient also reverses more rapidly with these smaller doses, “wake-up” time may be shorter, making this strategy beneficial for brief procedures. Importantly, however, clinicians must be aware that rapid changes in the brainstem’s concentration of opioid and sedative drugs markedly increase the potential for respiratory depression and apnea. As a practical point, this technique can be used only for ketamine administration because it causes markedly less respiratory depression than opioid and GABAergic drugs. This technique needs further study to delineate its safety and effectiveness and is suggested for consideration only by clinicians with extensive experience in ED PSA (Fig. 19.3).
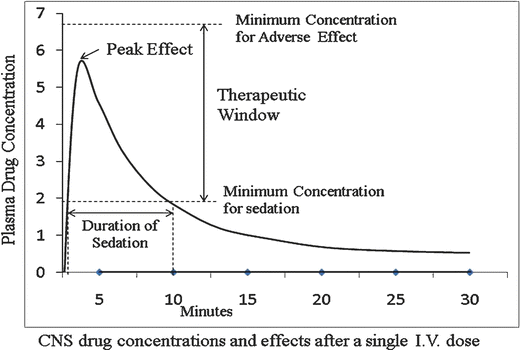

Fig. 19.3
Plasma drug concentration and CNS drug concentrations and effects after a single IV dose
A drug’s therapeutic window is used to describe the difference between the dose of that drug that results in the desired sedative or analgesic effect and the dose that results in adverse effects. A drug with a wide therapeutic window has a greater margin of safety for use for ED PSA. For example, accidental administration of a tenfold greater-than-intended dose of ketamine will likely result in prolonged recovery but relatively little cardiopulmonary depression [156], whereas the same error with propofol will result in apnea and hypotension [157].
Many reasonable medication options exist for ED PSA [78, 158]. Use of analgesic medication when pain is the primary cause of distress is the key, and balancing analgesia with anxiolysis makes sedations more pleasant for patients. For nonpainful procedures when immobility is the primary objective, sedative–hypnotic medications may be chosen. It is recommended that the clinician initially become familiar with a few specific agents or combination of agents that provide the desired effects of analgesia, sedation, and/or anxiolysis. Limiting one’s experience to a few agents better enables one to anticipate and manage adverse effects and events associated with those agents. One’s pharmacologic armamentarium then can be gradually increased and refined with tailoring of regimens to a specific patient’s characteristics. The following section summarizes medication effects and pharmacology in healthy children. Abnormalities in renal and hepatic function can significantly alter these parameters, particularly the duration of effects. In addition, significant variability in effect may occur between individuals due to genetically determined factors such as differences in drug receptor sites, metabolic activation, or clearance. Patients with ASA physical status Class III and higher also have less physiological reserves and therefore are more likely to have adverse effects with smaller doses.
Dosing Details
Titration to Desired Effect
Careful intravenous “titration” of medications using repeatedly administered small doses to achieve the desired clinical effect enables the practitioner to use the smallest effective dose and reduce the peril of oversedation with its increasing risks of respiratory depression and aspiration, and, furthermore, hasten recovery [70, 98, 103, 159]. Individual variation in sensitivity to the medication can also be detected; thus a smaller-than-expected dose may be found adequate for a given individual.
Knowledge of the time to peak effect of the specific medication is necessary to avoid “stacking” of doses when first gaining experience with titration. That is, if, to achieve deeper sedation, a subsequent dose is administered before the peak effect of the preceding dose has occurred, deeper-than-intended sedation can easily occur. For example, morphine has a peak effect of approximately 10 min. If an additional dose of morphine is administered after 5 min because the patient is still in significant pain, by 15 min after the original dose, when both the first and second doses are near peak effects, the patient may have significant respiratory depression due to an excessive accumulative dose. For this reason, titration is difficult with drugs that have longer than 1–3 min to peak effect time.
When a “typical” total dose for a specific procedure is known, that total dose may be divided and the increments administered at intervals shorter than “the time to peak effect” without likely overshoot. This strategy of repeated administration of fractional doses for fixed dose protocols—e.g., half of the anticipated total dose administered twice with administration separated by a short interval—reduces the risk for significant respiratory depression induced by some agents such as the combined technique using fentanyl and midazolam. This approach is suggested for providers who have less experience with a specific medication.
Intravenous Administration at the Hub
Injecting medications at or near the hub of the indwelling venous catheter allows one to know more precisely when the drug enters circulation and when the entire dose has been administered. This can avoid unintended continued infusion of residual drug in the intravenous tubing when adverse effects are occurring.
Intramuscular Administration
While IM administration avoids the need for placement of an IV catheter, it still requires a feared needlestick and makes titration to effect difficult. More importantly, if a serious adverse event occurs (e.g., severe laryngospasm), an emergent IV for resuscitation medications or fluids may be difficult to place. Specifically, ketamine-administered IM has been shown to be effective in achieving sedation. However, the IM route requires either use of a dose large enough to sedate all children—e.g., 4 mg/kg, which will oversedate some and result in greater frequency of adverse events [135]—or painful repeat administration of a smaller dose if the original dose is insufficient. Since the onset of IM ketamine is 5–15 min, titration without oversedation is difficult. Due to the large dose typically administered IM, recovery is prolonged [160].
Sedative–Hypnotic Agents
Commonly used sedative–hypnotic medications for procedural sedation include barbiturates, chloral hydrate, propofol, and etomidate. These drugs induce general depression of the central nervous system (CNS) by stimulation of inhibitory gamma-aminobutyric acid (GABA) receptors or other mechanisms that are not yet fully elucidated. None of these drugs have an analgesic effect. While deeply induced sedation (e.g., with propofol) may enable painful procedures to be accomplished, lighter sedation with less respiratory depression may be facilitated by the addition of an analgesic agent as described in subsequent sections. This chapter will review the common sedatives used in the ED with particular focus on their clinical applications and supporting literature from the speciality. (Refer to Chap. 9.)
Chloral Hydrate [78]
Indications: Chloral hydrate may be used to provide effective ED PSA in children less than 2 years of age, including those with congenital cardiac anomalies, who are undergoing painless diagnostic studies such as CT and MRI scans. Sedation is achieved in >80 % of young children. Chloral hydrate should not be considered a first-line agent in children older than 48 months because of decreased efficacy as compared with younger children. The drug may be administered orally or rectally. The oral preparation has a bitter taste that frequently requires administration in a flavored vehicle to disguise its taste; approximately a third of children may vomit soon after oral administration.
Contraindications/cautions/adverse effects: Children receiving chloral hydrate should be properly monitored and managed by appropriately trained personnel due to the risk of respiratory depression and hypoxia. Chloral hydrate should not be used in children with neurodevelopmental disorders due to an increased incidence of adverse effects and decreased efficacy as compared with healthy children. Chloral hydrate has the potential for resedation and may produce residual effects up to 24 h after administration. The elimination half-life is age dependent, with much longer effects in infants. These effects may occur long after the procedure is finished; reports describe infant deaths due to slumping in car seats with obstruction of the airway after discharge. Many infants may have unsteady gait, hyperactivity, or irritability the day after sedation. Other adverse effects include respiratory depression, hypotension, paradoxical excitement (0–15 %), vomiting (10–30 %), and, rarely, hepatic failure, areflexia, jaundice, gastrointestinal hemorrhage, and esophageal stricture [78, 161, 162]. These disadvantages along with its highly variable effects on older children and inherent difficulty with titration of oral medications make this agent less than ideal for children older than 1–2 years of age. Interestingly, children who have been fasted may have increased PSA failure rates. See Mace et al. for further details on dosing and adverse effects [78].
Pregnancy Category C
Dose: PO or PR; 50–125 mg/kg; typical initial dose 75 mg/kg. A second dose may be given, if needed, to a maximum of 2 g or 100–125 mg/kg total dose.
Onset/duration: sedation within 30–60 min, recovery by 60–120 min.
Mechanism of action: halogenated hydrocarbon with sedative–hypnotic but no analgesic effects.
Metabolization: rapidly metabolized by hepatic alcohol dehydrogenase to its active compound trichloroethanol and subsequently excreted in the urine [155]. The elimination half-life is age dependent: 40 h in preterm infant, 28 h in term infant, and 6–8 h in toddler.
Barbiturates
Barbiturates are pure sedatives with no analgesic effect. They potentiate the effect of GABA, the principal inhibitory neurotransmitter in the CNS, by binding to the GABAA receptor and prolonging the open time of the membrane chloride ion channel. In addition, barbiturates block the excitatory AMPA receptor [155].
Methohexital (Brevital®)
Indications: Methohexital administered by either the intravenous, intramuscular, or rectal route can provide effective sedation for children undergoing painless diagnostic studies such as CT or MRI scans. However, because of the readily induced respiratory depression associated with this medication, methohexital has not been used or studied extensively for procedural sedation in children, and thus its use should be considered only by experienced and knowledgeable clinicians.
Adverse effects: Respiratory depression and apnea are dose- and infusion rate dependent and are readily induced with intravenous administration but may occur with any route of administration. Hangover-like residual effects may last for 24 h.
Pregnancy Category B
Dosages: 1 mg/kg IV, 10 mg/kg IM, 25 mg/kg P.R.
Mechanism of action: ultrashort-acting, highly lipid-soluble barbiturate with rapid CNS uptake and redistribution. It has marked sedative–hypnotic but no analgesic effects.
Metabolization: Hepatic degradation with renal excretion results in an elimination half-life of 3.5 h and less accumulation of drug in body tissues compared to other barbiturates.
Pentobarbital (Nembutal®)
Indications: Pentobarbital is a short-acting barbiturate that induces relative immobility and can be safely used to sedate children to facilitate nonpainful diagnostic studies such as CT and MRI scans; redundant but supportive measures may include head positioning, supplemental oxygen, and occasional bag–valve–mask ventilatory support [158]. Pentobarbital successfully sedates >97 % of children for CT or MRI scans with higher success rates in children younger than 8 years of age [166–168]. Pentobarbital is more effective in providing sedation than midazolam [169] or etomidate [170] and causes fewer adverse respiratory events than propofol [171]. The addition of midazolam with pentobarbital does not appear to increase success rates and prolongs time to discharge [167].
Oral pentobarbital (4 mg/kg) has been found similar to oral chloral hydrate (50 mg/kg) in time to sedation and length of sedation; overall adverse event rate, including oxygen desaturation, was slightly lower with pentobarbital (0.5 %) than with chloral hydrate (2.7 %) [172, 173]. Of note, a database review found infants younger than 12 months of age sedated for elective CT or MRI with PO pentobarbital (4–8 mg/kg) had comparable effectiveness and fewer respiratory complications compared with IV pentobarbital (2–6 mg/kg); time to sedation was slightly longer with PO than with IV pentobarbital (18 versus 7 min), but time to discharge (~1 h 45 min) was similar. Total adverse event rate was similar (0.8 % [PO] versus 1.3 % [IV]), but oxygen desaturation was slightly more frequent for IV (0.2 % [PO] versus 0.9 % [IV]). Sedation effectiveness was comparable (99.5 % [PO] versus 99.7 % [IV]), leading the authors to recommend consideration of PO administration for this age group, even when an IV is in place [174]. In a randomized comparison of IV pentobarbital (maximum 5 mg/kg in incremental doses) or oral chloral hydrate (75 mg/kg) prior to MRI, children who received pentobarbital had a higher incidence of paradoxical reaction (14 % versus 9 %) and prolonged recovery with a similar failure rate [173].
Adverse effects: Respiratory depression is dose- and infusion rate dependent and is generally less than that seen with equivalently sedating doses of opioids or chloral hydrate [172, 173, 175]. Mild respiratory depression is usually seen at doses required for hypnotic effect. The following adverse events and frequencies have been reported: transient respiratory depression with oxygen desaturation of ≥10 % below the baseline in 1–8 %; vomiting in ≤1 % [167, 176, 177]; increased airway secretions, airway obstruction, coughing, and bronchospasm [166–168, 172, 176–178]; emergence reactions (hyperactivity in 5–7 %) [176, 178] of 8.4 % in children older than 8 years [178]; and paradoxical reaction (sustained inconsolability and severe irritability and combativeness for more than 30 min) in 0.01 % with oral pentobarbital [172] and in 1.5 % with intravenous pentobarbital [167]. Up to 35 % of children will have increased sleeping or hangover-like effects in the 24 h following pentobarbital sedation [172, 178]. Pentobarbital should be avoided in children with porphyria.
Pregnancy Category D
Dosages: IV (protocol used by author)—first dose, 2.5 mg/kg; if needed, subsequent doses, 1.25 mg/kg, may repeat × 3 to maximum of 7.5 mg/kg or 200 mg maximum.
IM: 2–6 mg/kg, to a maximum of 100 mg.
PO or PR (<4 years): 3–6 mg/kg, to a maximum of 100 mg.
PO or PR (>4 years): 1.5–3 mg/kg, to a maximum of 100 mg.
Onset/duration: The onset of action is related to the route of administration and subsequent absorption. The duration of hypnotic effect is dependent upon redistribution with recovery occurring within 50–75 min after IV or IM administration, even though the biologic half-life in plasma is 15–20 h [175].
After IV administration: sedation by 1–10 min (peak by 5–10 min), recovery by 1–4 h; most patients awakening within 30–60 min. [167, 169]
After IM administration: sedation by 10–30 min, recovery by 2–4 h.
After PO administration: sedation by 15–60 min, recovery by 2–4 h.
Mechanism of action: short-acting barbiturate with sedative–hypnotic but no analgesic effects; it induces relative immobility through nonselective depression of the CNS via facilitation of GABA receptors.
Metabolization: hepatic degradation with elimination half-life 15–20 h [175]. This may explain why many parents note it may take their children up to a day to return to normal behavior.
Anxiolytic–Amnestic–Sedative Agents
Benzodiazepines
Benzodiazepines produce a range of hypnotic (sedative), anxiolytic, amnestic, anticonvulsant, and muscle relaxant effects via modulation of the GABAA receptor, the most common inhibitory receptor within the brain. The GABAA receptor is composed of five subunits, each of which has multiple subtypes. The varying combinations of subunit subtypes result in different pharmacological and clinical effects (Table 19.6). When benzodiazepine binds to its site on the GABA receptor, it causes the receptor to have a much higher affinity for the GABA neurotransmitter. This results in the associated chloride ion channel opening more frequently, causing the neuronal membrane to become hyperpolarized [155]. Benzodiazepines have no analgesic effect. Benzodiazepines administered without other medications rarely cause severe adverse effects [179]. However, when benzodiazepines are combined with other drugs such as opiates, marked respiratory depression and apnea can readily occur [98]. Midazolam (Versed®) and diazepam (Valium®) are commonly used benzodiazepines for procedural sedation because of their shorter duration and potent anxiolytic and amnestic effects.
Table 19.6
Comparison of benzodiazepines
Drug | Dose (mg/kg) | Onset (min) | Peak effect (min) | Duration (h) |
|---|---|---|---|---|
Midazolam | 0.05–0.15 | 1–3 | 3–5 | 0.5–1 |
Diazepam | 0.1–0.2 | 1.5–3 | 1–2 | 2–6 |
Lorazepam | 0.03–0.05 | 1–5 | 3–4 |
Paradoxical Reactions
Severe behavioral changes, typically during recovery, resulting from benzodiazepines as well as barbiturates have been reported including mania, anger, and impulsivity. Individuals with borderline personality disorder appear to have a greater risk of experiencing severe behavioral or psychiatric disturbances from benzodiazepines. Paradoxical rage reactions from benzodiazepines are thought to be due to partial deterioration from consciousness, generating automatic behaviors, fixation amnesia, and aggressiveness from disinhibition with a possible serotonergic mechanism playing a role [180, 181]. In the context of ED PSA, parents should be forewarned about the possibility of excitability, increased anxiety, and agitation in response to midazolam. Recommendations for management of this adverse effect include protecting patients from self-harm while allowing further recovery, deepening sedation with fentanyl or diphenhydramine or administration of caffeine [180, 182].
Midazolam (Versed®)
Indications: Midazolam is a water-soluble benzodiazepine that induces anxiolysis and mild sedation. Most children will not fall asleep with midazolam alone, even at higher doses. Consider another agent or combine with another agent (e.g., pentobarbital) if procedure requires patient to remain motionless (e.g., MRI scan). Midazolam has more potent amnestic effects, quicker onset, and shorter duration of action compared to diazepam [183–186]. Since it is water soluble, midazolam can be administered intramuscularly, as well as PO, IV, or intranasally (IN). Midazolam may be used for seizure control but longer-lasting agents such as lorazepam are typically used. Midazolam also has antiemetic effects, an additional benefit when coadministered with opioids or ketamine [187].
Contraindications/cautions/adverse effects: Midazolam causes minimal hemodynamic effects (mild hypotension with compensatory tachycardia) but dose- and infusion rate-dependent respiratory depression and apnea occur when midazolam is administered in concert with opioids [98]. An important adverse reaction to benzodiazepines in children is the disinhibitory reaction, possibly mediated by central cholinergic mechanisms [180]. Paradoxical excitement or dysphoria during recovery may be increased in older children when midazolam is coadministered with ketamine [89].
Pregnancy Category D
Dosages: IV/IM, anxiolysis, 0.05 mg/kg IV with maximum of 2 mg; sedation, 0.1 mg/kg IV with maximum of 5–10 mg. If titrating to effect, administer doses at 3 min or greater intervals to avoid stacking effects. However, the anticipated dose (e.g., 0.1 mg/kg) may be divided and administered at 1–2 min intervals to reduce respiratory depression.
PO: 0.2–0.75 mg/kg.
IN: 0.2–0.4 mg/kg (use 5 mg/mL IV solution to reduce volume, use atomizer, or drip slowly); more rapid onset and shorter duration than oral. When administered with an atomizer device, this technique is well tolerated and effective to achieve mild to moderate sedation [188]. If the intravenous solution is dripped into the nares without atomization, most children complain of a burning sensation [189–191].
Onset/duration:
IV: sedation within 1 min, peak effect by 2–6 min, recovery by 30–60 min. [194]
IM: sedation within 5–15 min, peaks by 30 min, recovery by 30–60 min. [195]
PO: anxiolysis and mild sedation peak within 15–20 min, recovery by 60–90 min. [189]
IN: effect within 5–10 min, duration 45–60 min. Use of atomizer results in faster onset.
Mechanism of action: See benzodiazepine introduction.
Metabolization: Midazolam is degraded almost completely by cytochrome P450-3A4 in the liver and excreted in the urine. Midazolam metabolites have little CNS activity, unlike those of diazepam.
Reversal: Midazolam-induced apnea or respiratory depression may be counteracted by administration of flumazenil 0.01–0.04 mg/kg (maximum 0.5 mg) IV over 30 s and repeated every 60 s to desired response. A cumulative dose of 3 mg may be necessary. Flumazenil may reverse midazolam-induced hypnotic and amnestic effects but not ventilatory depression [127]. The patient must be closely monitored, typically for 2 h after flumazenil administration, for resedation and respiratory depression. Recurrence of sedation has been reported in up to 7 % of cases, most commonly in children under 5 years of age [128]. Flumazenil may cause seizures in patients chronically on benzodiazepine medications and should be used cautiously in patients on medications that can lower seizure threshold.
Diazepam (Valium®)
Indications: Diazepam has excellent antianxiety, skeletal muscle relaxation, and amnestic properties, but because its duration of effect is longer than that of midazolam, diazepam is seldom used for ED PSA or preprocedure anxiolysis. It is considered 2–4 times less potent than midazolam.
Contraindications/cautions/adverse effects: Drowsiness may last 2–6 h with resedation occurring at 6–8 h due to enterohepatic recirculation and formation of active metabolites. Like other benzodiazepines, diazepam readily causes respiratory depression with rapid administration.
Diazepam’s propylene glycol carrier causes burning sensations on intramuscular and intravenous injection and erratic absorption with intramuscular administration. Administer with caution in patients with liver and kidney dysfunction.
Pregnancy Category D
Dosages: IV, 0.04–0.2 mg/kg/dose q 2–4 h. PR: 0.5 mg/kg/dose.
PO: 0.12–0.8 mg/kg.
Onset/duration: IV, within 1.5–3 min. PR: 7–15 min.
PO: 30–60 min.
Mechanism of action: See benzodiazepine introduction.
Metabolization: Diazepam undergoes hepatic microsomal oxidation with renal excretion. Liver and kidney dysfunction, as well as active metabolites including desmethyldiazepam and oxazepam, may prolong effects.
Other Non-analgesic Sedative Agents
Propofol (Diprivan®)
Propofol is a sedative hypnotic agent with no analgesic properties [155]. It is the most commonly used parenteral agent for induction and maintenance of general anesthesia in the United States, due in large part to rapid and pleasant recovery from anesthesia induced by this potent agent [155]. Little or no nausea is associated with propofol and its amnestic effect is similar to that from midazolam [196]. Many adults and older children remark on awakening that they feel as if they have just had a good nap. These characteristics have resulted in propofol’s rapid increase in popularity as an agent for scheduled [88, 197] and ED PSA for children [158, 198].
Propofol, however, has a narrow therapeutic window, which makes PSA titration to desired effect without oversedation more difficult than with many other agents. Significant respiratory depression and hypotension are relatively common (see “Adverse Effects” section) [88, 199]. Propofol can be used alone for painless procedures such as MRI or CT scans, or, at greater doses, for painful procedures. However, because significant respiratory depression or apnea is associated with doses necessary for painful procedures, smaller doses of propofol have been combined with analgesic opiates or ketamine for ED PSA [199–201]. Although combining ketamine with propofol may have theoretical benefit by using lower doses of each agent to reduce the undesirable adverse effects of both agents, a 2007 review of published studies in adults and children found the combination had not demonstrated superior clinical efficacy compared with propofol alone. Studies conflicted regarding reduced hemodynamic and respiratory adverse effects with the combination compared with propofol monotherapy [202]. A comparison of propofol + ketamine to propofol + fentanyl for PSA in toddlers undergoing burn dressing changes found similar minimal impact on blood pressure and respiratory rate but less restlessness with the addition of ketamine [203].
Use of propofol for ED PSA should be preceded by specific training and supervised experience. It is recommended that when propofol is administered, an experienced provider with advanced airway skills be dedicated to administering the sedation and managing the airway and cardiorespiratory status of the patient. In-depth knowledge of adverse effects and advanced airway skills are essential for safe use of this drug.
Pharmacology
The exact mechanism(s) by which propofol exerts global CNS depression has not been fully elucidated. However, there is evidence that propofol potentiates GABAA receptor activity by slowing the channel-closing time, with lesser effects on GABAB receptors, modestly inhibits the N-methyl-d-aspartate (NMDA) receptor, modulates calcium influx through slow calcium-ion channels, and locks sodium channels [204].
Pharmacokinetics [157]
Propofol is highly lipophilic and rapidly diffuses from plasma into body tissues, particularly the highly perfused brain. The onset of action of propofol as determined by time to unconsciousness (i.e., loss of response to voice command) is within 1 arm–brain circulation time (the time required for the drug to travel from the site of injection to the site of action in the brain) and can be as brief as 15–30 s, but is more typically 40–60 s, dependent upon the rate of administration. Since propofol is rapidly distributed from CNS to inactive storage sites such as muscle and fat, recovery from anesthesia is rapid with duration of action about 5–10 min. The short duration of sedation after repeated doses can be explained by rapid metabolic clearance from blood and slow redistribution of the drug from the peripheral tissues. Thus, the pharmacokinetics of propofol after IV administration are best described by a 3-compartment model with rapid distribution of the drug from blood into the brain and other tissues, rapid metabolic clearance from blood, and slow redistribution of the drug from the peripheral compartment back into the blood stream, resulting in sub-hypnotic plasma levels of drug.
Propofol is rapidly and extensively metabolized in the liver to less active conjugates, which are excreted mainly in the urine. Since plasma clearance exceeds hepatic blood flow, it appears that the drug also is metabolized at extrahepatic sites. Mean total body clearance of propofol appears to be proportional to body weight; obese patients have a substantially higher body clearance than leaner individuals.
Indications: Propofol sedation of children in the ED has been reported primarily for fracture reduction with fentanyl, morphine, or ketamine coadministered [199–201, 205]. Sedation or distress scores were low during fracture reduction with propofol + morphine or fentanyl and similar to ketamine + midazolam or morphine + midazolam [200, 201]. Mean recovery times after propofol for these studies were 15–23 min. Unlike other PSA techniques, with the exception of nitrous oxide, repeated or continuous dosing of propofol causes little prolongation of recovery when administered for less than 1–2 h. Thus, after longer procedures, such as complex laceration repair or emergent MRI scans during which either repeated doses or continuous infusion of propofol is required, recovery typically is still within 15–30 min. [206]
Contraindications/Cautions/Adverse Effects: Transient respiratory depression, apnea, upper airway obstruction, or laryngospasm may occur in many patients, especially during induction of sedation [88, 199, 207]. A recent study suggests that the administration of induction dosages of propofol slowly over 3 min decreases the incidence of respiratory depression [208]. Increasing upper airway narrowing due to muscle relaxation, especially at the level of the epiglottis, has been shown with increasing depth of propofol sedation/anesthesia [209]. Loss of protective airway reflexes during apneic periods may place patients at increased risk of pulmonary aspiration as the ensuing bag–mask positive-pressure ventilation increases gastric pressure and risk of passive regurgitation [88]. Therefore, candidates for propofol sedation must be carefully screened for risks of “full stomachs,” URIs, and difficult airways [210]. These events are frequent enough when sedating with propofol that many providers routinely administer supplemental oxygen and monitor with end-tidal capnography, in addition to having a functioning anesthesia or CPAP ventilation bag at the bedside [107, 108, 117].
The main adverse cardiovascular effect of propofol is hypotension, in part related to decreases in peripheral vascular resistance [157, 211]. In spontaneously breathing patients, as much as a 30 % decrease in blood pressure may be seen with little or no changes in heart rate [205, 212]. The decrease in blood pressure is dose- and infusion rate dependent and is potentiated by coadministration of opioids such as fentanyl [211, 213]. Propofol may rarely induce profound bradycardia and cardiac arrest in hypovolemic patients or in those at risk for hypotension or with cardiac dysfunction [88, 214]. Administration of additional fluids and a cautious rate of IV infusion may help reduce the risk of propofol-induced hypotension.
Because of the increased risk of apnea and hypotension compared to other agents for PSA, many providers avoid use of propofol in ED patients determined to have difficult airways, cardiac dysfunction, brief fasting, or ASA physical status Class 3, 4, or 5 [117, 199].
Propofol is formulated as an emulsion in soybean oil, glycerol, and purified egg products because it is essentially insoluble in aqueous solutions. Propofol therefore cannot be administered to patients with allergies to eggs or soy. In addition, to inhibit bacterial growth, some preparations contain sodium metabisulfite, which may cause allergic-type reactions in susceptible individuals, including anaphylaxis and life-threatening or less severe asthmatic episodes [157].
Despite the addition of disodium EDTA or sodium metabisulfite to inhibit bacterial growth, significant bacterial contamination of open containers has been associated with serious patient infection. Using aseptic technique, propofol should be administered shortly after removal from sterile packaging [155].
Injection site pain is common with propofol but often may not be recalled due to propofol’s amnestic effects. In ED PSA, coadministration of morphine or fentanyl for procedural analgesia may reduce this effect [117]. Lidocaine 0.5 mg/kg administered intravenously immediately prior to propofol infusion and use of large antecubital veins also may help ameliorate this minor adverse effect [157, 200].
Involuntary movement (myoclonus) has been reported in 15–20 % of pediatric patients undergoing propofol anesthesia, typically during induction [157]. Myoclonus significant enough to interrupt the procedure, the majority of which were radiological, however, occurred only at a rate of 2/10,000 in elective sedations with propofol [88].
Pregnancy Category B
Dosages: Propofol can be administered intravenously in doses of 1–2 mg/kg to achieve sedation. Note, however, administration of 2–3.5 mg/kg followed by continuous infusion of 100–300 mg/kg/min is commonly used for induction of general anesthesia [117, 199–201, 205, 215, 216].
Published studies of pediatric ED PSA for fracture reduction used an initial bolus of 1 mg/kg propofol administered over 1–2 min followed by additional doses of 0.5 mg/kg every 1–3 min based on patient response [199, 201, 205]. Mean total propofol doses in these studies were 2.5–4.5 mg/kg. Alternatively, one study followed the initial 1 mg/kg bolus immediately with a propofol infusion at 67–100 mg/kg/min until cast completion; most children required an additional bolus of propofol during the infusion to achieve the desired level of sedation [200]. In each of these studies, propofol was administered shortly after morphine or fentanyl administration.
Administration [157]: Commercially available 1 % propofol injectable emulsion (10 mg/mL) may be used without dilution. If dilution is necessary, the drug may be diluted with 5 % dextrose injection to a concentration of not less than 0.2 % (2 mg/mL) in order to maintain the emulsion. Propofol should be discarded if there is evidence of separation of the emulsion. The emulsion should be shaken well just prior to administration.
Using aseptic technique, contents of a vial may be transferred into a sterile, single-use syringe and administered shortly after removal from sterile packaging. The manufacturers state that propofol is compatible with several IV fluids (e.g., 5 % dextrose, 5 % dextrose and lactated Ringer’s, 5 % dextrose, and 0.2 or 0.45 % sodium chloride) when a Y-type administration set is used.
Etomidate
Indications: Etomidate has potent hypnotic (sedative) and amnestic but no analgesic effects. It is in an aqueous solution of propylene glycol; therefore, burning on injection is a common complaint. Since etomidate rapidly induces unconsciousness with little hemodynamic effect and clinical recovery occurs within minutes, it is frequently used in the emergency setting to induce unconsciousness prior to neuromuscular blockade during endotracheal intubation [217–219].
Recent reports suggest etomidate may be safe and effective for brief nonpainful procedures such as CT scans and can be combined with fentanyl for fracture reductions. Early reports were inconclusive about the safety and effectiveness of etomidate for ED PSA in children [158, 220–223]. However, a small study of ED pediatric patients sedated for head and neck CT found successful completion of the CT in 57 % with etomidate doses up to 0.3 mg/kg and 76 % with doses up to 0.4 mg/kg, in contrast to a success rate of 97 % for pentobarbital [170]. Etomidate 0.2 mg/kg IV was infused over 30 s, with additional doses, if needed, of 0.1 mg/kg IV over 30 s at 1 min intervals, to a maximum total dose of 0.4 mg/kg. Duration of sedation was 13 min and parents felt their children returned to normal behavior much earlier than with pentobarbital. A more rapid infusion technique in another study reported a 99 % successful completion of CT scans with etomidate in 446 fasted ASA-PS Class I and II children; duration of sedation was 34 min [224]. With a proximal tourniquet in place, 0.5 mg/kg lidocaine (maximum dose 25 mg) was first administered through the intravenous catheter to mitigate burning from the subsequent etomidate infusion, a “mini-Bier block” technique. After 1 min, the tourniquet was removed and etomidate 0.3 mg/kg was infused over 2–3 s. If sedation was not adequate, an additional 0.15 mg/kg bolus was administered within 1 min of the initial dose. If needed, an additional 0.15 mg/kg bolus was given during scans requiring multiple views or repositioning. Median total etomidate dose was 3.3 mg/kg. With this technique, 1 patient had apnea and the CT scan was not completed; otherwise significant respiratory depression did not occur. Although most of these children were not ED patients, it suggests this agent may be used successfully for this purpose.
For fracture reduction, etomidate 0.2 mg/kg infused intravenously over 60–90 s resulted in effective sedation in 92 % of children compared to 36 % with midazolam 0.1 mg/kg IV [225]. Both were combined with fentanyl 1 mg/kg IV. Median recovery time in those reaching adequate sedation was 12 min with etomidate and 24 min with midazolam. Desaturation occurred in 22 % of children in both groups; all responded quickly to free flow oxygen administration or head repositioning; no patient experienced apnea or required positive-pressure ventilation. Myoclonus occurred in 22 % of patients who received etomidate, but it was described as mild and brief and did not interfere with the fracture reduction. Pain on injection of etomidate was noted in 46 % of children. Further studies of etomidate are needed to define better safety and efficacy parameters for PSA, particularly in unfasted emergency patients.
Contraindications/cautions/adverse effects: Similar to midazolam, transient apnea with rapid infusion may rarely occur when etomidate is administered alone [224], but respiratory depression may occur in 20 % or more of children receiving etomidate coadministered with fentanyl or morphine [225]. Pain with injection in 2–20 % and myoclonus in 8–40 % of patients are associated with etomidate infusion [221, 226, 227]. When present, myoclonus that can resemble seizures usually lasts less than 1 min and can be decreased by the coadministration redundant of other drugs. These tremors are benign and not epileptiform activity [226, 228].
Although trials investigating etomidate-induced adrenal suppression associated with PSA in noncritically ill children are not available, studies in adults and children have demonstrated cortisol depression for up to 24 h with as little as a single dose of etomidate. This suppression may be clinically significant in patients with hemorrhagic or septic shock, leading some to suggest consideration of alternative agents or to combine etomidate with glucocorticoids for induction of unconsciousness for tracheal intubation or PSA in these patients [229–232].
Pregnancy Category D
Dosages: 0.2–0.3 mg/kg IV.
Onset/duration: onset of sedation within 30–60 s, with duration of deep sedation 3–12 min when using a dose of 0.2–0.3 mg/kg [71]. Sufficient recovery for discharge may take 30–45 min. [224]
Mechanism of action: Etomidate, like propofol, is structurally unrelated to other anesthetics. It is an imidazole derivative that is thought to induce sedation through enhanced gamma-aminobutyric acid (GABA) neurotransmission [155].
Metabolization: Etomidate is highly protein bound in blood and is degraded by hepatic and plasma esterases to inactive products. It exhibits a bi-exponential decline, with a redistribution half-life of 2–5 min and an elimination half-life of 68–75 min [155].
Sedative–Analgesic Agents
The following are primary analgesic agents. Sedation generally requires higher doses of opioids or addition of sedative–hypnotic agents, both of which significantly increase respiratory depression. Ketamine induces sedation and amnesia but opioid agents cause little amnesia.
Opiates (Narcotics) (Table 19.7): Fentanyl (Sublimaze®)
Table 19.7
Comparison of opioid medications
Opioid | IV dose (mg/kg) | Peak | Duration |
|---|---|---|---|
Fentanyl | 0.001–0.002 (1–2 μg/kg) | 30–60 s | 30 min |
Morphine | 0.1 | 10 min | 4–5 h |
Meperidine | 1 | 10 min | 2–4 h |
Indications: Fentanyl is a high-potency synthetic opiate with minimal hemodynamic effects. Due to its lipophilic nature and rapid biphasic redistribution, onset of analgesia and sedation occur rapidly with intravenous administration but are of short duration, making it a favorable agent for ED PSA. Fentanyl, by weight, is 80–100 times more potent than morphine. It provides significant analgesia and mild sedation for painful procedures but is not recommended for anxiety control or for control of spontaneous movement. Since fentanyl, unlike morphine, does not cause clinically significant histamine release, it is the opiate of choice in patients who have increased potential for hypotension, e.g., trauma or sepsis [233].
Fentanyl has been administered in oral lozenges (oral transmucosal fentanyl citrate (OTFC)) for ED PSA for laceration repair. However, titration to effect is difficult with this technique and it has been associated with frequent nausea, vomiting (20–50 %), and pruritus [234–237]. OTFC has also been used for rapid (30 min) analgesia in children with fractures [238].
Of note, atomized intranasal administration of fentanyl in children in acute pain in the ED has been shown to provide significant pain relief by 5–10 min [239, 240]. One small study of children 1–4 years old undergoing suturing in the ED found intranasal sufentanil, a more potent analog of fentanyl, plus midazolam provided sedation by 20 min without vomiting or other significant adverse events [241]. Further study is needed to clarify safety and efficacy of atomized intranasal techniques for ED PSA.
Fentanyl plus midazolam: A primary goal with most painful ED PSA is attenuated or blocked unpleasant recall of the procedure. Since fentanyl induces minimal amnesia and cannot completely block procedure-related pain without extreme respiratory depression, it is typically combined with midazolam to induce amnesia for residual procedural pain. Although the combination of fentanyl and midazolam can cause significant respiratory depression [98], both agents have competitive antagonists that readily reverse undesirable effects. If titrated carefully, a small dose of naloxone of 1 mg/kg will reverse respiratory depression but retain much of the analgesia effect. This reversibility makes this combined technique an optimum and frequently used approach for ED PSA [158].
The dose of midazolam that maximizes amnestic effect is not well established. Furthermore, while the onset of peak amnestic effect is indistinct, the duration of action appears to be fairly long, hence a broad window within which to administer the analgesic agent, fentanyl. Thus, it is recommended to maximize the capability to administer sufficient amnestic agent by infusing the midazolam before the fentanyl is given, since the synergistic respiratory depressant effects of the two medications may limit the ability to administer sufficient amnestic agent if it is given after the fentanyl.
Adequate analgesia for painful procedures always requires sufficient narcotic to cause some degree of respiratory depression (assuming narcotic naive patients). Use of local anesthesia for the procedure (e.g., a hematoma block for fracture reduction) can significantly reduce the amount of systemic analgesic agent needed and thus reduce respiratory depression. It is important to time the “peak analgesia effect” (peak brain concentration) with “maximal analgesia need” (at time of the maximally painful part of the procedure); hence the analgesic agent is administered after the amnestic agent. The respiratory depression is typically counteracted by the pain of the procedure. Particular attention to ventilatory sufficiency should occur after the painful procedural stimulus ends, since respiratory depressant effects will persist for minutes to hours after the last dose of medication [124]. This adverse effect may be exacerbated by oral or parenteral opioid analgesics administered prior to the PSA.
Contraindications/cautions/adverse effects: Fentanyl, like other opioid analgesics, causes dose- and infusion rate-dependent respiratory depression characterized by decreases in respiratory rate, tidal volume, minute ventilation, and ventilatory response to carbon dioxide. Hypotension and bradycardia may also occur with rapid infusion or larger doses. Although return to relative alertness typically occurs within 20–30 min after IV administration, respiratory depressant effects may last several hours. Patients may be awake but need to be reminded to breathe due to the drug’s depression of the brainstem response to rising plasma CO [120, 124, 242].
Respiratory depression can be lessened by administering the expected total dose in divided amounts, e.g., 0.5 mg/kg/dose, and infusing each dose over 30–60 s at 1–2 min intervals. Respiratory depression is markedly increased by coadministration of sedative–hypnotic medications such as midazolam or barbiturates [9, 98]. At the level of deep sedation, many children will have respiratory depression or partial upper airway obstruction due to muscle relaxation and may require airway-opening maneuvers, supplemental oxygen, or painful stimulation [9].
Respiratory depression is readily reversed by the competitive antagonist naloxone. Titration of naloxone in small doses of 1 μg/kg stopping at the endpoint of reversal of respiratory depression will retain much of the analgesia effect. Repeated doses may be necessary as respiratory effects may outlast the reversal effects of naloxone. Administration of a “full” dose of naloxone may cause significant pain, hypertension, tachycardia, vomiting, and other undesirable adverse effects.
Chest wall rigidity may occur with rapid infusion of large doses (usually >5 mg/kg), especially in infants. This life-threatening adverse effect will manifest by lack of spontaneous chest wall movement, dropping oxygen saturations, and an inability to ventilate the patient with positive pressure by bag and mask. Reversal with naloxone or paralysis with succinylcholine may be needed to manage this adverse event.
Pregnancy Category C
Dosages: for analgesia; 1–2 μg/kg, intravenously. Titrate to effect by administering doses of 0.5 μg/kg over 15–30 s, repeated every 1–2 min. A total dose of 1–2 μg/kg usually can be administered without causing significant respiratory depression, unless coadministered with midazolam. For significantly painful injuries, an initial dose of 1 μg/kg usually may be administered safely over 30 s.
For ED PSA: fentanyl + midazolam; midazolam, 0.05–0.1 mg/kg intravenously over 1–2 min, is administered first, titrated to an endpoint of drooping eyelids and slurred speech. A total dose of 10 mg likely is sufficient for amnesia in large adolescents. Then fentanyl, 0.5 μg/kg intravenously over 30 s, is repeated to an endpoint of decreased patient responsiveness to a relevant painful stimulus such as squeezing the fracture site or palpating the abscess. If local anesthesia is used for the procedure, approximately 1 μg/kg fentanyl may be sufficient. For intensely painful procedures, such as fracture reduction without a hematoma block, up to 2 μg/kg may be necessary [9]. Respiratory depression is likely at this dose; therefore, time the end titration of fentanyl as the painful part of the procedure is begun; the procedure-related pain will stimulate the patient and counteract some of the respiratory depression. Additional doses of fentanyl may be administered after about 10 min if the patient becomes agitated or manifests significant pain during longer procedures.
Fentanyl comes in 2 mL vials of 50 μg/mL. Titration is easier and safer if the concentrated fentanyl is diluted to 10 μg/mL by adding 2 mL of fentanyl to 8 mL of normal saline, resulting in 10 mL of 10 μg/mL.
Onset/duration: Analgesia with mild sedation after IV administration of fentanyl is within 30–60 s, with greatest sedative–analgesic effects lasting 5–10 min. Although return to relative alertness typically occurs within 20–30 min after IV administration, respiratory depressant effects may last several hours. Patients may be awake but “forget to breathe” due to the drug’s depression of the brainstem response to rising plasma CO2 [120, 124, 242].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree