Sedation measures
• Adverse events related to sedation
• Adverse events related to procedure
• Procedure completion rate
• Procedure times
• Patient recovery times
• Patient satisfaction
• Parent satisfaction
• Provider satisfaction
• Cost
• Speed of recovery of cognition
• Speed of recovery of locomotion
Pre-procedure Preparation and Patient Assessment
Sedation for pediatric gastrointestinal procedures should be tailored to a patient’s physical status. This is most typically done in accordance with guidelines from the American Society of Anesthesiologists (ASA) [32–35]. Increased use of electronic medical records has improved the potential for providers to identify and manage patients with complex medical histories before procedures are performed [36]. In pediatrics and in adults, consideration of the patient’s age, medical condition (ASA level), and developmental status is often critical. Data suggests that the smallest and youngest pediatric patients with the highest ASA classifications are at greatest risk for complications during gastrointestinal procedures [1, 37].
When working with children undergoing gastrointestinal procedures, it has been noted that personality and psychosocial development stages may vary widely and impact a child’s response to sedatives, both in terms of the rapidity of effect and the depth achieved [38, 39]. Patients can be roughly divided into four different age groups: less than 6 months, greater than 6 months, school aged (4–11 years), and adolescents. Infants under 6 months of age may have little anxiety and tend to sedate easily. Infants greater than 6 months who have developed “stranger anxiety” may respond best if parents remain next to them during the induction of sedation. School-aged children manifest “concrete thinking” and may be surprisingly difficult to sedate, as they tend to conceal high anxiety levels [10]. Adolescents also may appear composed during pre-procedure preparations, and then become disinhibited and anxious after initial doses of sedatives.
Especially in school-aged children, a relaxed, detailed, and reassuring discussion of what to expect during the procedure, including the insertion of an intravenous catheter (IV) may decrease patient anxiety levels [39]. The use of topical anesthetics for IV insertion such as topical lidocaine cream, or oral anxiolytics, such as midazolam, may be warranted [20, 40]. Children who exhibit greater distress during the IV insertion have been shown to experience significantly greater distress and pain throughout the rest of the procedure [40].
Regardless of sedation regimens employed, it is essential to perform airway assessments at every step of the endoscopic process, beginning with the pre-procedure evaluation and concluding in the recovery room. All providers who care for children with gastrointestinal disorders should be schooled in airway assessment, including anesthesiologists, gastroenterologists, and nurses [3]. There is increasing interest among gastroenterologists in understanding how best to assess a patient airway using standardized methods, such as the Mallampati score [41] (Table 18.2, Fig. 18.1).
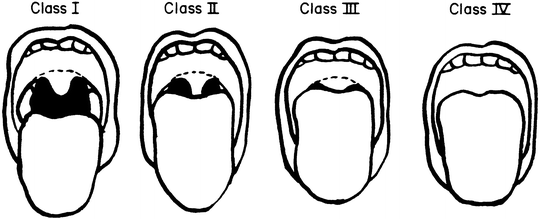
Mallampati score |
|---|
• Class I: Uvula is completely visible |
• Class II: Partially visible uvula |
• Class III: Soft palate visible but not uvula |
• Class IV: Hard palate visible only, not soft or uvula |

Fig. 18.1
Mallampati classification of pharyngeal structures (Reprinted with permission from Figs. 1 and 2, page 488. Samsoon GL, Young JR. Difficult tracheal intubation: A retrospective study. Anaesthesia. 1987 May;42(5):487-490.)
It also may be particularly important to identify patients at risk for obstructive sleep apnea, as they are considered at high risk for sedation-related complications [42]. Many such patients are obese, which along with hypertension, diabetes, and heart disease may function as independent risk factors for hypoxemia and other complications during gastrointestinal procedures [43]. “STOP-BANG” is a validated bedside screening instrument that has been used by anesthesiologists to predict the risk of unrecognized obstructive sleep apnea in adults [44, 45] (Table 18.3). In one prospective study, Cote et al. demonstrated that more than 40 % of adults presenting for screening colonoscopies at a US academic medical center meet criteria for a positive STOP-BANG score [44]. (Refer to Chaps. 4 and 7.) To date, there is no literature to support the validity of the STOP-BANG tool for pediatrics and modifications would need to be made to adapt it for use in children. Obese patients at risk for obstructive sleep apnea may specifically benefit from advanced monitoring or from tailored sedation regimens. One such regimen known as “Target-Controlled Infusions” (TCI) utilizes computers to achieve goal drug concentrations and modifies dosages using physiologic feedback [46, 47]. (Refer to Chap. 31.) It is unknown whether application of the STOP-BANG tool is appropriate in children. Nevertheless, it is an irrefutable fact that obesity and associated comorbidities, such as obstructive sleep apnea, are increasingly common in pediatric populations, and it may be reasonable to assume that obese children have similar risk profiles to obese adults.
Table 18.3
STOP-BANG scoring modela
S | Snoring: Do you snore loudly (louder than talking or loud enough to be heard through closed doors)? | Yes | No |
T | Tired: Do you often feel tired, fatigued, or sleepy during the daytime? | Yes | No |
O | Observed: Has anyone observed you stop breathing during your sleep? | Yes | No |
P | Blood pressure: Do you have or are you being treated for high blood pressure? | Yes | No |
B | BMI: BMI more than 35 kg/m2 | Yes | No |
A | Age: Age over 50 years | Yes | No |
N | Neck circumference: Neck circumference greater than 40 cm | Yes | No |
G | Gender: Male | Yes | No |
Beyond airway assessment, a careful review of patient gastrointestinal conditions, past medical history, as well as prior experiences with sedation and procedures guides the triage of a child undergoing a gastrointestinal procedure. Some gastrointestinal disorders increase the riskiness of the procedure [48]. In particular, upper gastrointestinal bleeds, anatomic or physiologic obstruction of the upper gastrointestinal tract, recent ingestion of blood or food, and septic patients who need common bile duct clearance all will place a patient at higher risk for complications both from the procedures and from the sedation [49]. Premature infants as well as older children with body mass indices (BMI) for age greater than the 85th percentile may also be at increased risk [50, 51]. In addition, patients with significant liver disease or cirrhosis may be at increased risk of sedation-related complications, including respiratory compromise and delayed recovery, as well as psychometric deterioration and even encephalopathy, due to altered drug clearance [52, 53].
Patient Positioning
All patients undergoing diagnostic upper and lower endoscopic procedures with sedation should be placed in the left lateral decubitus position [35]. This is because patients who are placed in the supine position are more susceptible to pooling of secretions in oral pharynx, and risk upper airway obstruction or laryngospasm. Patients undergoing ERCP may require a prone or prone-oblique position [48]. Obviously, airway monitoring and management is more challenging for patients in prone positions and they may require advanced monitoring.
Common IV Sedation Regimens for Pediatric Gastrointestinal Procedures
Table 18.4 lists commonly used sedative regimens for pediatric gastrointestinal procedures. In general, the most common moderate sedation regimens used for pediatric endoscopy combine a narcotic analgesic (e.g., meperidine or fentanyl) with a benzodiazepine (e.g., diazepam or midazolam) [18]. A brief review of the important pharmacokinetic, pharmacodynamic, and clinical properties of those medications most commonly used for GI sedation, directed to the GI concerns, will follow. Chapter 9: Pharmacology and Clinical Application of Sedatives, Analgesics, and Adjuncts provides a more thorough and detailed review of all sedative agents and adjuncts.
Table 18.4
Recommendations for dosages of drugs commonly used for IV sedation for pediatric gastrointestinal procedures *
Drug | Route | Maximum dose (mg/kg) | Time to onset (min) | Duration of action (min) |
|---|---|---|---|---|
Benzodiazepines | ||||
Diazepam | IV | 0.1–0.3 | 1–3 | 15–30 |
Rectal | 0.2–0.3 | 2–10 | 15–30 | |
Midazolam | Oral | 0.5–0.75 | 15–30 | 60–90 |
IV | 0.05–0.15 | 2–3 | 45–60 | |
Rectal | 0.5–0.75 | 10–30 | 60–90 | |
Opioids | ||||
Meperidine | IV | 1–3 | <5 | 120–240 |
IM | 1–3 | 10–15 | 120–180 | |
Fentanyl | IV | 0.001–0.005 (1–5 μg/kg in 0.5–1.0 μg/kg increments) | 2–3 | 30–60 |
Ketamine | IV | 1–3 | 1 | 15–60 |
IM | 2–10 | 3–5 | 15–150 | |
Fentanyl
As a fat-soluble narcotic that rapidly penetrates the blood–brain barrier, fentanyl is considerably more potent and fast acting than both morphine and meperidine. Its onset of action is about 30 s after IV administration, and its opioid effects last about 30–45 min. Intravenous fentanyl should always be administered slowly, as it has been associated with the dangerous side effects of chest wall and glottic rigidity after rapid administration [54].
Fentanyl is variably metabolized by the liver, especially in young children. Delayed fentanyl excretion has been reported in neonates with compromised hepatic blood flow [55]. Several studies have suggested that fentanyl may not represent an ideal sedative for infants. In particular, it has been associated with significant apnea in infants less than 3 months of age [56]. These unique pharmacokinetics of fentanyl are certainly relevant to the performance of pediatric endoscopy. In particular, fentanyl’s termination of action occurs with redistribution of drug metabolites from the plasma, rather than from metabolism, causing its potential respiratory depressive effects to outlast its opioid effects. Fentanyl should be administered to children in small increments, allowing for a minimum of several minutes between doses.
Midazolam
Midazolam is a benzodiazepine that is 3–6 times more potent than diazepam. It may be administered by many routes: IV, oral, rectal, intramuscular, and intranasal. When administered IV, the onset of action is 1–5 min, with peak effect achieved at 30 min to 1 h. Several early pharmacokinetic studies have bolstered evidence that midazolam may be metabolized and excreted more rapidly in children than adults [20, 57, 58]. Midazolam is relatively unique among benzodiazepines in that its clearance appears to be dose-related, with increased clearance at escalating dosage [59]. Pediatric gastroenterologists have reported the need to require larger weight-adjusted doses for pediatric versus adult patients in order to achieve similar doses and duration of sedation [60].
Reversal Agents for Narcotics and Benzodiazepines
Reversal agents are available only for benzodiazepines and narcotics. Table 18.5 lists reversal agents and their recommended dosages for children. Although reversal agents have been used in adults to expedite recovery, it is important to recognize that there may be resedation as the effect of sedative outlasts that of the reversal agent [33]. Most endoscopy and pediatric sedation guidelines stipulate that patients who receive a dose of a reversal agent should be monitored for an extended period and administered repeat doses if necessary [35, 61].
Table 18.5
Reversal agents for benzodiazepines and opioids and recommended dosages *
Drug | Class | Route | Dose (mg/kg) | Time to onset (min) action (min) | Duration of antagonist |
|---|---|---|---|---|---|
Flumazenil | Benzodiazepines | IV (max 3 mg/h) | 0.01 | 1–2 | <60 |
Naloxone | Narcotics | IV/IM | 0.1 | 2–5 | 20–60 |
Ketamine
Ketamine is a dissociative agent that largely spares upper airway muscular tone and laryngeal reflexes, and may represent an alternative to narcotics and benzodiazepines for sedating children for gastrointestinal procedures [62–66]. Ketamine may also be useful for sedating patients who are opioid tolerant [67]. As a derivative of phencyclidine, ketamine binds to opiate receptors, and rapidly induces a trancelike cataleptic condition with significant analgesia. Routes of administration include oral or rectal, although intravenous or intramuscular is a more common route of administration during endoscopy.
Unlike most sedatives, ketamine is almost always effective at significantly immobilizing patients with minimal cardiac and respiratory effects, and is considered to have a broad margin of safety. It should be used with caution in patients less than 3 months of age, as well as those with histories of airway instability, tracheal abnormalities, active pulmonary disease, cardiovascular disease, head injury, central nervous system masses, hydrocephalus, porphyria, and thyroid disease [68–70].
Ketamine is considered by many to be contraindicated in patients with a history of psychosis [69, 70]. To date, its main drawback has been its association with hallucinogenic emergence reactions in some children [71, 72]. Its administration may be partnered with that of a short-acting benzodiazepine, such as midazolam. Brecelj et al. demonstrated in a single-blind randomized controlled trial that premedication with 0.1 mg of midazolam IV (2.5 mg maximum dose midazolam) prior to ketamine may reduce the frequency of emergence reactions [73]. However, outcomes of this approach have not been consistent, and some data suggest that midazolam may actually increase agitation in postpubertal children [74, 75]. Ketamine has also been recently reported to be successfully combined with other agents, including analgesics, such as fentanyl and tramadol [76], remifentanil [77], and even dexmedetomidine [78], to provide excellent regimens for a variety of endoscopic procedures.
Ketamine has been associated with increased airway secretions and an increased incidence of postoperative nausea and vomiting. During upper endoscopy, ketamine has been associated with a potential for laryngospasm [30, 63, 79]. Attempts to minimize this risk by administering anticholinergics may not be successful. Indeed, matched case–control analysis of 8,282 ketamine procedures in the emergency department revealed no association between age, dose, procedure, medical status, route of delivery, and the administration of anticholinergics with the occurrence of laryngospasm [64].
Nevertheless, the question remains whether ketamine is superior enough to replace the traditional moderate sedation regimens for pediatric endoscopy (opioids and benzodiazepines). A retrospective review of 402 endoscopic procedures with different sedation combinations reported that a combination of midazolam and ketamine was both safe and superior (by physician report) to such traditional sedation regimens [79]. However, more recent data collected by independent observers suggests that ketamine is associated with a comparably higher rate of laryngospasm and similar incidence of patient movement and need for restraint than do those sedated with midazolam and fentanyl [30]. These findings may substantiate those who have suggested that ketamine may be most appropriately used for liver biopsy, a very brief procedure with minimal upper airway stimulation [69].
Nitrous Oxide
Nitrous oxide is an inhalational gaseous mixture that has analgesic, sedative, and amnestic properties. It is generally prepared as 50 % nitrous oxide in oxygen, and is a short-acting agent with rapid onset of action (3–5 min) and short duration of effects after withdrawal (3–5 min). Several studies have suggested that nitrous oxide may provide rapid and effective sedation for children undergoing gastrointestinal procedures, without inducing deep sedation [80, 81]. Nitrous oxide may be adequate for procedures that do not induce pain, such as upper gastrointestinal endoscopy and flexible sigmoidoscopy. Comparisons of nitrous oxide with opioid and benzodiazepine regimens for more uncomfortable procedures have had conflicting results: Forbes et al. have found that nitrous oxide may not provide enough analgesia for colonoscopy [81], while Mcculloch et al. have reported that nitrous oxide is as effective as IV midazolam and pethidine at relieving pain and bloating, while minimizing cardiopulmonary risks in elderly patients [82].
Propofol
Propofol is an ultra-short-acting anesthetic that features both a rapid onset of action and a short recovery time. It can be used to induce and maintain a spectrum of sedation levels, as well as to achieve anesthesia. Investigation with Functional Near-Infrared Spectroscopy (fNIRS) has shown that drug-related effects on cerebral hemodynamic activity are dose dependent, with decreased oxygenation of the dorsolateral prefrontal cortex during bolus infusions and deeper levels of sedation [83]. Studies of propofol in healthy volunteers have found that sedation with propofol alone allows esophageal intubation at the beginning of a procedure, and that recovery from both induced loss of consciousness and respiratory compromise occurs within 3–4 min of stopping an infusion [84].
Propofol may be administered during pediatric endoscopy either as a total intravenous anesthetic or in combination with other sedatives, including inhalational agents [24, 85]. Propofol, alone or in combination with other agents, has been shown in multiple studies to be highly effective at inducing sedation in children who are undergoing both upper and lower endoscopy, and provides excellent amnesia for the procedure [86–90]. Several recent studies have also suggested it may be a preferable agent in patients with significant liver disease or cirrhosis [52, 53].
Currently, many pediatric gastroenterologists, both in the United States and abroad, use anesthesiologist-administered propofol as a primary means of sedation [1]. This trend has paralleled the performance of pediatric GI procedures in dedicated endoscopy units as a means of decreasing the need for operating room time [89, 91, 92]. Although the use of propofol by anesthesiologists in dedicated endoscopy units may offer scheduling advantages, it is not clear that this practice changes day-to-day efficiency. Indeed, while children who receive propofol have shorter induction times than children who received midazolam and fentanyl, use of propofol compared with more traditional intravenous regimens has not been shown to improve unit throughput times [85].
A main pharmacologic disadvantage of propofol is its relatively narrow therapeutic range. Pharmacokinetic studies of children who received propofol demonstrate that average total propofol doses per kilogram of body weight to achieve targeted plasma propofol concentrations are higher in younger children [93, 94]. Propofol also has a high propensity to cause hypotension. One recent study has suggested that use of diluted propofol may significantly reduce sedation-related hypotension and other adverse events, without affecting its potential to provide deep sedation [95].
Propofol can be given alone or in combination with other sedatives. Elitsur et al. reviewed propofol sedation for endoscopic procedures in children and found that a lower propofol dosage was needed when propofol was given in combination with midazolam and fentanyl than when propofol was given alone [96]. Propofol was also found to confer amnestic effects, independently of those conferred by midazolam.
Titrating propofol to achieve sedation without inducing general anesthesia requires clinical expertise and, even when administered by anesthesiologists, carries the risk of inducing anesthesia rather than sedation. Kaddu et al. reported transient apnea in 20 % of pediatric patients receiving anesthesiologist-administered propofol for upper endoscopy [89]. A retrospective review of 176 children in Thailand, 175 of whom received propofol delivered by anesthesia personnel or an anesthesiologists, reported one patient who required an unanticipated endotracheal intubation [97]. Slow (not rapid) administration of propofol (over 3 min) may confer less respiratory depression [98].
Non-anesthesiologist-Administered Propofol Sedation
Non-anesthesiologist-administered propofol sedation (NAAPS) is an acronym used to describe the administration of propofol under the direction of a physician by an appropriately qualified registered nurse or physician who has not been trained as an anesthesiologist [99–101]. Multiple NAAPS protocols have been developed, and all have stressed a curriculum of drug knowledge, as well as specific practical skills in airway management [101, 102]. Nurse-administered propofol sedation (NAPS) involves administration of propofol by dedicated nurses in the endoscopy unit, and has been described as successful by groups in many countries across the world, after implementation of special training programs [103].
Although there is little reported data of either NAAPS in children, one prospective study described a protocol of 1–2 mg/kg of propofol induction dose followed by 0.5–1.0 mg/kg supplements that was administered by pediatric residents [104]. The sedation providers had been specifically trained in cardiopulmonary resuscitation via a 4-week training period during which they had performed bag-mask ventilation and endotracheal intubation a minimum of 20 times. Patients were limited to ASA I and ASA II. Those with any indication of airway obstruction (existing or potential), respiratory disease, seizures, or risk of aspiration were excluded. Overall there was a 0.7 % (6/811) incidence of positive pressure ventilation, brief oxygen desaturation in 12 %, and no occurrence of endotracheal intubation. Although this study was too small to adequately demonstrate the safety of NAAPS in children, it does substantiate the need for training and airway management skills.
NAAPS has been a controversial topic since its inception [105]. Generally speaking, gastroenterologists and their representative medical societies across the United States and Europe have repeatedly noted that in adults the administration of propofol and standard sedation regimens have been found to be comparable with respect to efficacy and reported rates of complications [106–108]. Nevertheless, a guideline in 2010 from the Center for Medicare and Medicaid Services (CMS) restricted the administration of deep sedation with propofol, in particular, without the presence of a clinician trained in anesthesia [109]. A major factor in this policy decision was the fact that non-anesthesiologists, such as gastroenterologists, are not specifically trained in the comprehensive skills required to care for patients along the entire continuum of sedation (Table 18.6). In 2011, 21 European National Societies of Anesthesia published a consensus statement that upheld the CMS statement that propofol should be administered only by those trained in the administration of general anesthesia [110]. At this time, propofol in children is recognized to have high potential to induce respiratory depression and cardiovascular instability and is essentially universally administered by anesthesiologists for pediatric endoscopy [1, 2].
Table 18.6
Continuum of depth of sedation. Definition of general anesthesia and levels of sedation/analgesiaa,*
Minimal sedation anxiolysis | Moderate sedation/analgesia (“conscious sedation”) | Deep sedation/analgesia | General anesthesia | |
|---|---|---|---|---|
Responsiveness | Normal response to verbal stimulation | Purposefulb response to verbal or tactile stimulation | Purposefulb response following repeated or painful stimulation | Unarousable even with painful stimulus |
Airway | Unaffected | No intervention required | Intervention may be required | Intervention often required |
Spontaneous ventilation | Unaffected | Adequate | May be inadequate | Frequently inadequate |
Cardiovascular function | Unaffected | Usually maintained | Usually maintained | May be impaired |
Dexmedetomidine
Dexmedetomidine is a highly selective alpha2-adrenoreceptor agonist with sedative, analgesic, and antisialagogue effects. In many ways, the drug profile of dexmedetomidine suggests it may be a good drug for pediatric endoscopic sedation, as it offers hemodynamic stability, and has minimal effects on respiration and cognitive function. Nevertheless, to date there has been little to no investigation of the efficacy, safety, or cost of this sedative for gastrointestinal procedures. A small prospective study used independent observers to evaluate the sedation of 50 patients in terms of vital signs, as well as patient and endoscopist satisfaction during upper endoscopy with either dexmedetomidine or midazolam [111]. Patients were not randomized, and only the independent observer was blinded, which limited the study’s conclusions. The results suggested that dexmedetomidine was superior as a sole sedation agent to midazolam. Another recent abstract publication prospectively randomized 231 adults to receive either propofol or dexmedetomidine and found both regimens comparably safe [112]. Future studies may help to identify clinical situations where use of dexmedetomidine may be preferable during endoscopic sedation.
Training in Sedation Administration
Regardless of sedative regimen used, performance of sedated gastrointestinal endoscopy in children requires a carefully coordinated team of physicians and nurses [35, 113]. Optimization of team performance may be enhanced through routine drills that involve high fidelity simulation, and allow a chance for teams to practice high-stakes patient management in a safe environment [114]. Generally speaking, GI clinicians find simulation to be enjoyable, valuable, and realistic to their practice. A multisociety sedation curriculum for gastrointestinal endoscopy was published in 2012 and recognizes the basic competencies in knowledge and performance that must be achieved by non-anesthesiology trainees [35]. Anesthesiologists who are involved with the administration of sedation for gastrointestinal procedures can benefit from developing specific skill sets, as well as a good understanding of the range and goals of endoscopic procedures [115–117].
Monitoring of Children Undergoing Endoscopic Procedures with Sedation
Generally speaking, the emphasis of patient monitoring during GI procedures is on ventilation—either by visual assessment or from physiologic monitors (pulse oximetry, precordial stethoscope, capnography). All team members need to work together to identify suboptimal ventilation and to employ appropriate timely interventions.
Pulse Oximetry
Although visual assessments are considered to be as important as electronic monitoring for ensuring patient safety, oxygen desaturation represents a particularly objective means of detecting poor respiratory effort in sedated children undergoing gastrointestinal procedures. (Refer to Chap. 2.) If a provider fails to detect suboptimal ventilation by clinical assessment, he/she will often intervene to stimulate patient respiration if a pulse oximeter detects minor desaturation. On the other hand, it is important to recognize that oxygen desaturation is a relatively late sign of suboptimal ventilation [50]. Furthermore, while supplemental oxygen during upper GI endoscopy has been shown to decrease the incidence of desaturation and increase the likelihood of achieving 100 % arterial oxygen saturation [118], it is critical to understand that even patients with supplemental oxygen may be poorly ventilating [3].
Capnography
The dilemma posed by relying on pulse oximetry for monitoring children during endoscopy is that patients may be well saturated despite having significant carbon dioxide retention. In the past decade, improved compact microstream capnographs with aspiration flow technology have allowed the accurate real-time graphic display of ventilatory waveforms in non-intubated patients [119]. (Refer to Chap. 6.) Employing capnography in the pediatric endoscopy setting may reveal that abnormal ventilation is occurring during procedures in children at rates higher than expected [120]. The ASA in 2009 released a statement entitled Statement on Respiratory Monitoring During Endoscopic Procedures, which suggests that capnography “be considered” [121]. (Refer to Chap. 2.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


